The pH scale
The pH scale Solutions can be neutral, acidic or alkaline. For example, pure water is neutral with a pH of 7. A solution of table salt (sodium chloride) is also neutral, so it has a pH of 7 too.
The pH scale is a number scale that goes from 0 to 14. It shows how acidic or alkaline a water-based solution is. The pH scale is used to sort solutions as acidic, alkaline or neutral.
Neutral solutions are exactly pH 7. Acidic solutions have pH numbers lower than 7. The lower the pH number, the more acidic a solution is. Alkaline solutions have pH numbers higher than 7. The higher the pH number, the more alkaline a solution is.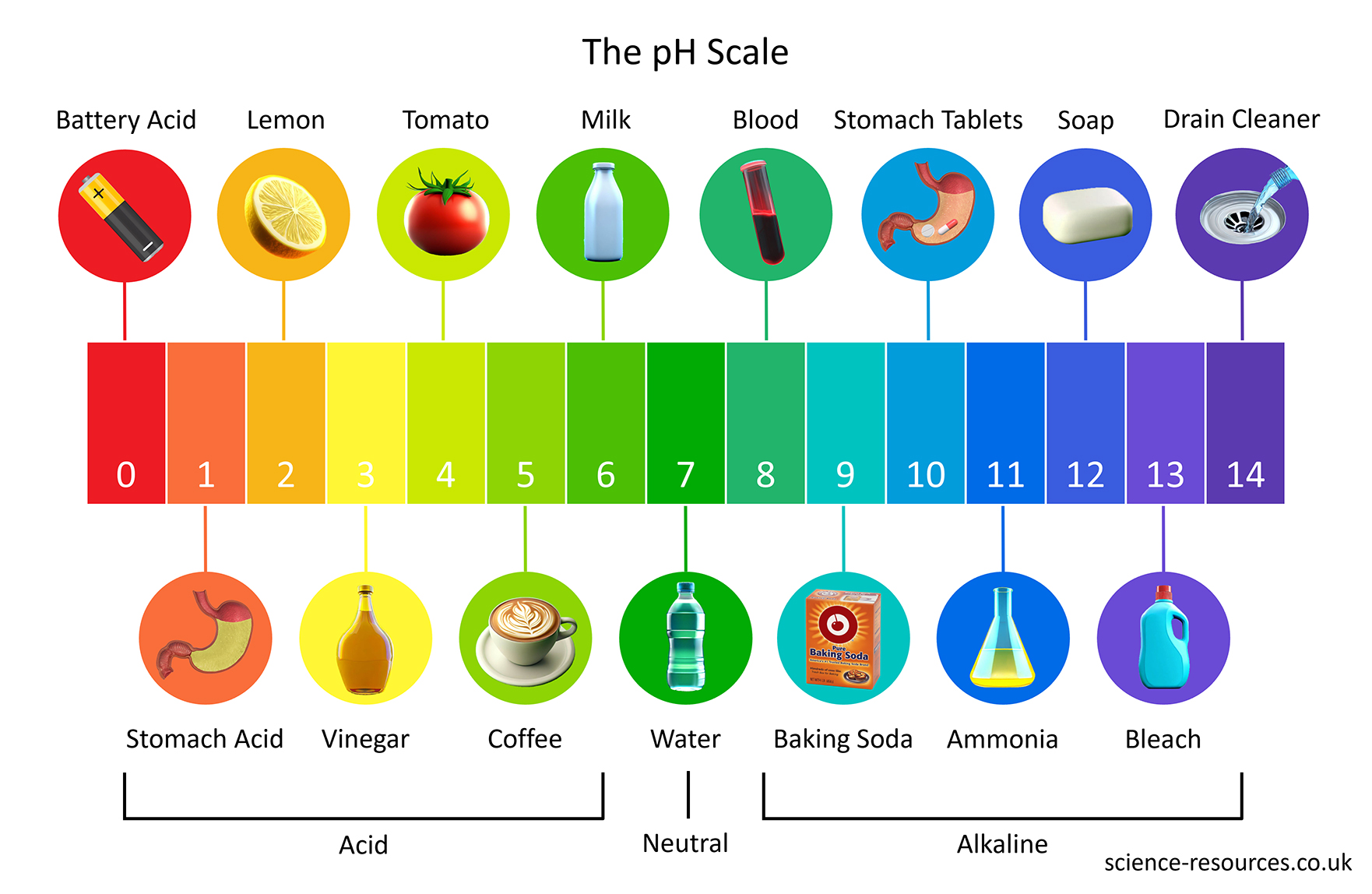
In science we often use the term water-based solution. It describes a solution that’s made of water. pH can only be measured in solutions that have water.
Therefore, we can measure how acidic or alkaline a substance is by dissolving it in water.
For example, copper sulfate is a solid that looks like blue crystals. When copper sulfate is mixed with pure water, we can measure the pH of the solution. The pH is about 6 which shows that copper sulfate solution is a bit acidic.
How do you measure the pH of a substance? A pH meter is a device that shows the pH value as a number. A pH meter can give an accurate measurement if it is well maintained. This means that the measurement is close to the true value of the pH. How to use an indicator How to use universal indicator
To measure the pH of a substance, it has to be an aqueous solution. This means that the substance is dissolved in water.
There are two ways to measure the pH of an aqueous solution:
An indicator is a substance that changes its colour according to the pH of the solution it is mixed with. Some indicators are in liquid form, so we can add a few drops of them to the solution we want to test. Other indicators are paper strips, and we can dip them into the solution.
Litmus is an example of an indicator. It is red in acidic solutions and blue in alkaline solutions.
Universal indicator is a special indicator because it has different colours for many different pH values. The colours of universal indicator for different pH values are shown below.
Universal indicator is a solution or a paper that contains a mixture of several different indicators. It can show us how acidic or alkaline a solution is, not just whether it is acidic or alkaline. It does this by using the pH scale, which goes from pH 0 to pH 14.
Universal indicator changes its colour depending on the pH of the solution. It is red for very acidic solutions and dark purple for very alkaline solutions. It is green for neutral solutions that have a pH of 7.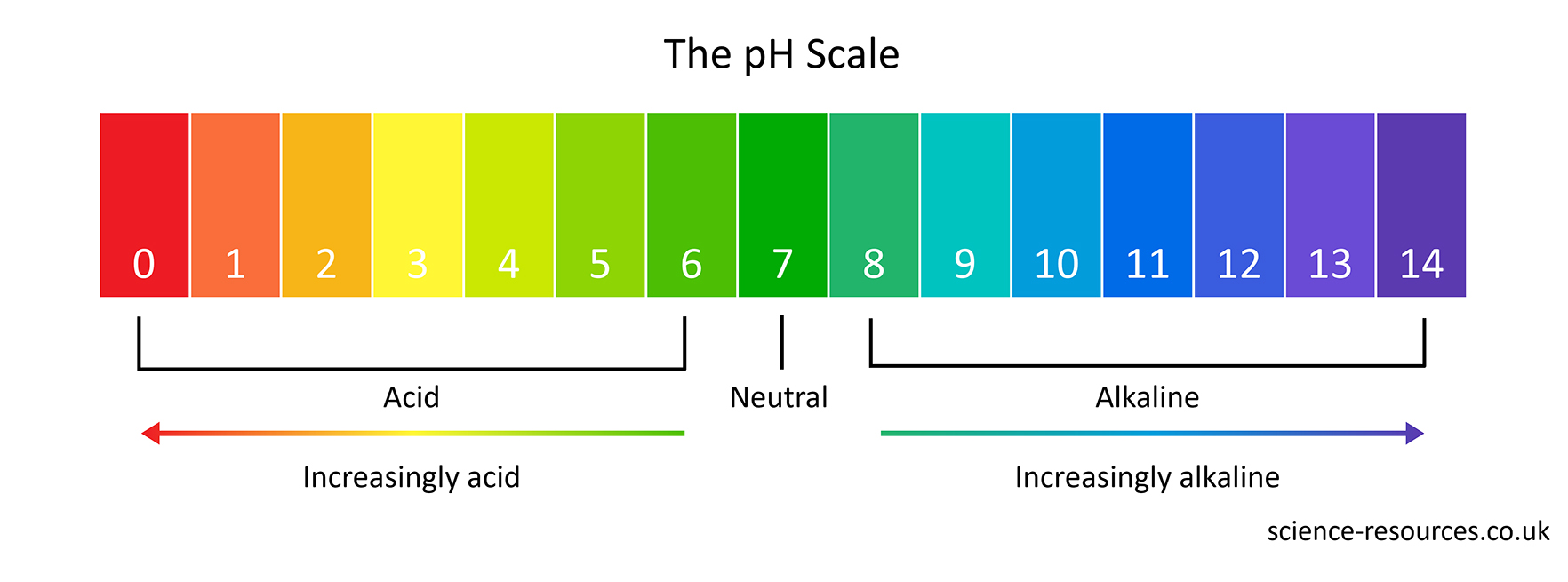
Summary: