Cracking Hydrocarbons
It
is when big hydrocarbon molecules are broken down into smaller ones. This
is done by heating the hydrocarbon in the presence
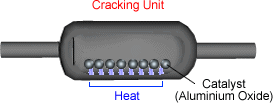
of a catalyst. This is a form of thermal decomposition. The catalyst helps to speed up the reaction. Hot vapour of hydrocarbons is passed over powdered aluminium oxide
catalyst at roughly 400ºC - 700ºC.
The long carbon chain molecules break apart or 'crack'
on the surface of the powered catalyst.
So
what is CRACKING?
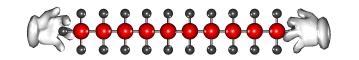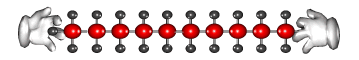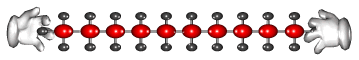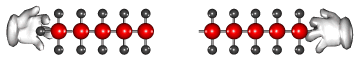
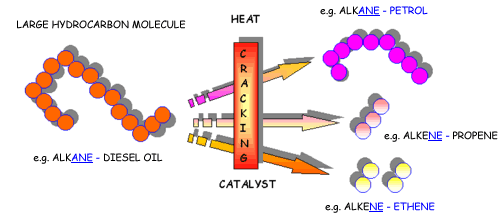
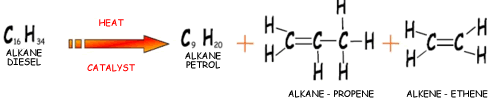
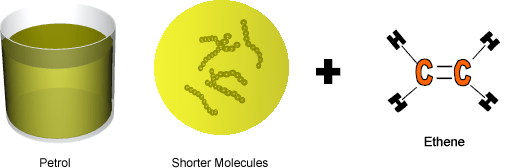
Cracking produces short-chain hydrocarbons called alkanes, like petrol and alkenes, like ethene and propene. These alkenes are very reactive due to their double bonds and therefore can be used to make other substances. Ethene, for example can be used to make ethanol and polythene.
![]()
Tags:Hydrocarbons, Cracking, Petrol, Ethane, Molecules, Diesel, Alkenes, the organic structure methane has what formula, Catalyst