Rates of Reaction
The speed at which a reaction
proceeds can be explained by using the collision theory. What must first happen
for a chemical reaction to take place? Well......................! Particles don't Collide Particles Collide but are not moving fast enough
Rates of Reaction
Collision Theory
(Kinetic Theory)
No Reaction
No Reaction
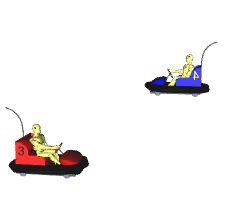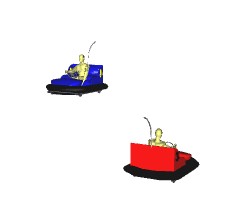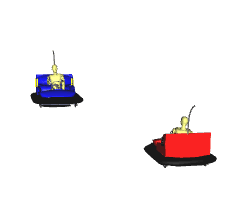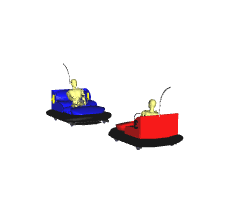
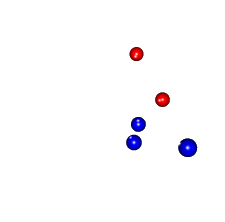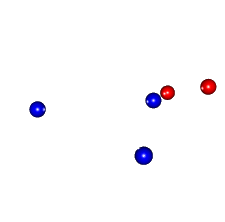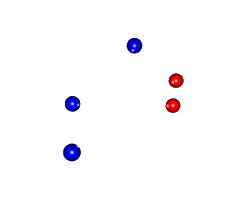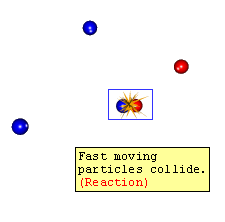
Fast moving Particles Collide
Reaction
1. The reacting particles must collide with each other 2. 'head on' 3. with enough energy (known as the activation energy) to break existing bonds 4. and with the correct orientation to bring reactive sites close together 5. to form new bonds |
 |
More Collisions Increase The Rate of Reaction The
rate of a Reaction Depends on Four Factors: 1. Concentration
- (or
PRESSURE FOR GASES) 2. Temperature 3. Catalyst 4. Size
of Particles
- (or
SURFACE AREA) Rise
in Concentration - (or
PRESSURE FOR GASES) increases the the number of collisions If the solution is made more concentrated (or pressure is increased in the case of gases), then the number of reacting particles are increased in a unit volume. Having more particles results in more
collisions, which leads to faster
a rate of reaction i.e. reaction
finishes in a quicker time. Change in temperature alters the kinetic energy of reacting particles and hence the number
of successful collisions with enough energy to break existing bonds and make product particles.
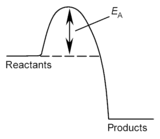
The minimum energy needed to break
existing bonds is called the ACTIVATION ENERGY
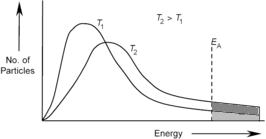
(EA). Increasing
the temperature of reacting particles results in: a) increases the range
of kinetic energies; b) increases the average
kinetic energy; c) increases the number
of particles with more than the activation energy (see graphs below). When the temperature of the reacting
particles is increased they all start
to move a lot faster. As a result
of their quikness they have more collisions. Relating The Theory To The Factors
Affecting Rate
Low
Concentration
High Concentration
Rise in Temperature
increases the number of collisions
Low
Temperature
High
Temperature
Tags:Rates of Reaction, Collision Theory, Kinetic Theory, Catalyst, Particle collision