Reactions of metals with acids
Metals and acids Metal + Acid → Salt + Hydrogen You can use the acronym M.A.S.H. to remember this general reaction. Reactivity of metals
Some metals and acids react to make a salt and hydrogen gas.
The metal gets smaller and smaller as it reacts with the acid.
You can see bubbles of gas coming out of the solution. The gas is hydrogen.
You can test this by using a lit splint. Hydrogen is flammable, so it will make a squeaky pop sound when it meets the flame. This proves that hydrogen is there.
Some metals react very easily. They make new substances quickly when they join in chemical reactions. Other metals react very slowly, and they do not join in chemical reactions easily.
We can make a list of the metals based on how easily they react. The most reactive metals are at the top of the list, and the least reactive metals are at the bottom of the list. This list is called the reactivity series.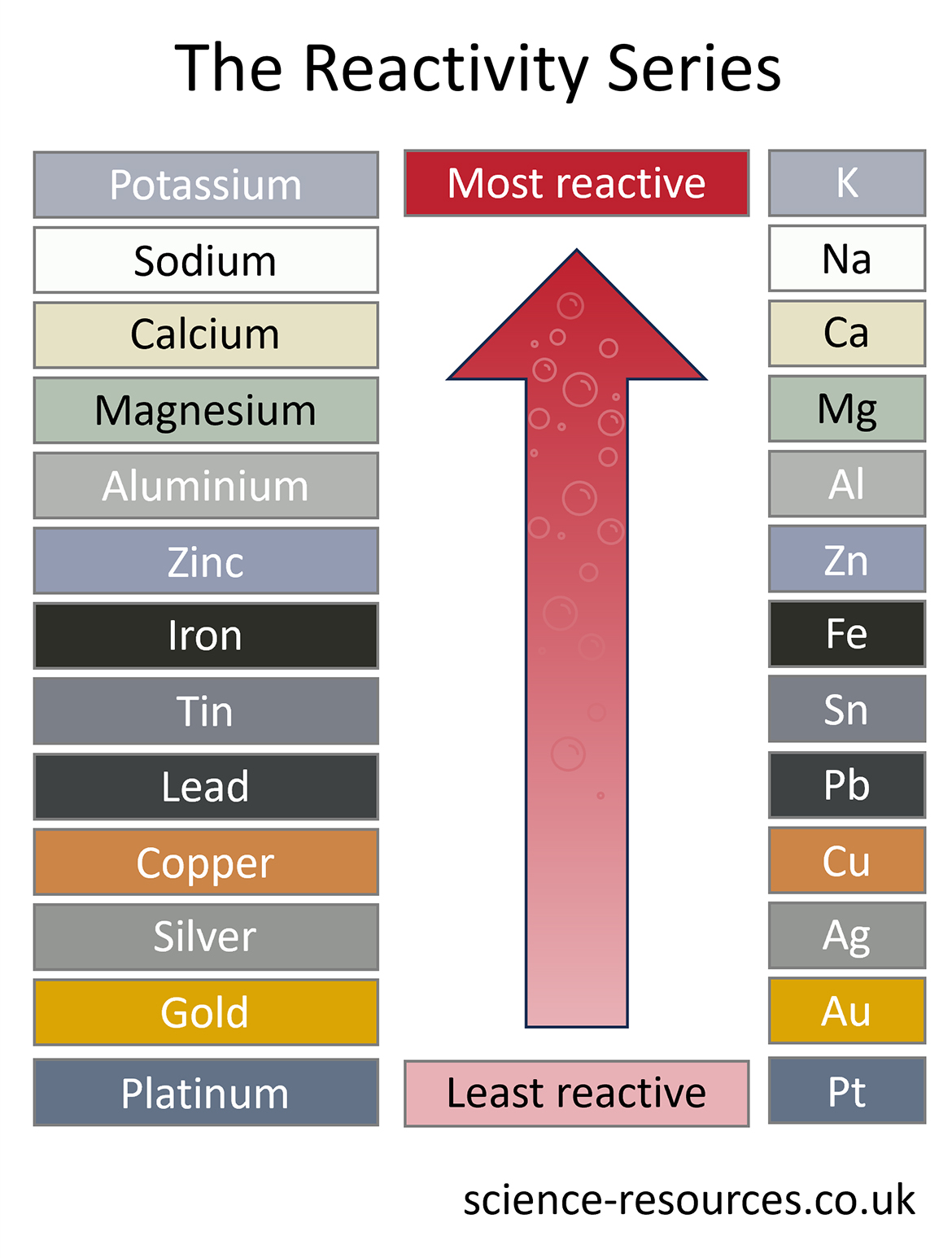
Naming the salt from the reaction of a metal and an acid 1. The first word is the name of the metal For example, if magnesium reacts with an acid, the salt will have magnesium as its first word. 2. The second word of the name is taken from the name of the acid Hydrochloric acid → chloride Nitric acid → nitrate Sulfuric acid → sulfate For example, zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen. The reaction of metals with acids can be described using the following chemical equation:
The name of the salt that is made from the reaction of a metal and an acid depends on the names of the metal and the acid.
Name of metal + name of acid → salt name
magnesium + sulfuric acid → magnesium sulfate + hydrogen
Or (symbol equation):
Mg + H2SO2 → MgSO2 + H2
Summary: