Elements, compounds and mixtures
Elements The periodic table Periodic table of elements
Elements An element is a substance that is made of only one kind of atom, and cannot be split into simpler substances. Some examples of elements are oxygen, hydrogen and iron. Some elements are single atoms, but some are molecules of the same kind of atom.
The periodic table shows all the 118 chemical elements. They are arranged on the table in a specific way.
The table has two main groups: metals and non-metals. Metals are on the left and in the centre, while non-metals are on the right. There is a line that zig-zags across the table to separate metals and non-metals. Hydrogen is an exception and is placed above the table, because it has a different atomic structure than other elements.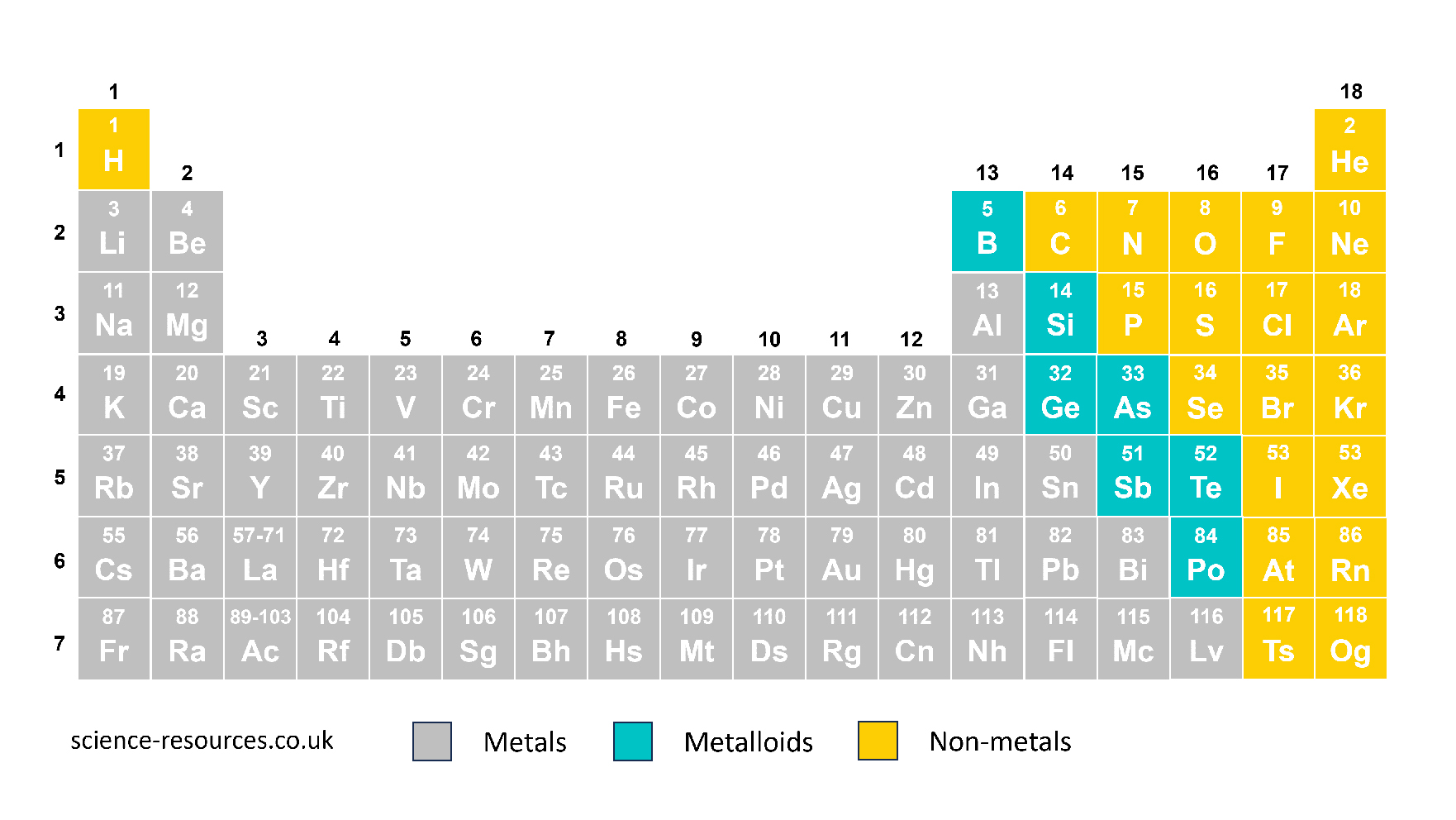
Hydrogen is the first element on the table of elements. It has the symbol H. It is a gas that explodes and makes a loud ‘squeaky pop’ sound when it meets a flame in a test tube. Helium is the second element on the table of elements, and its symbol is He. Helium is also a gas, but it is very different from hydrogen because it does not react with anything. Helium is used for party balloons because it is lighter than air. The first 94 elements on the table of elements, up to plutonium, are found naturally on Earth and in other places in the universe. The heaviest elements are made by humans using nuclear reactions, but they are not shown on this version of the table of elements.
Compounds Mixtures In a mixture, the two ingredients can be separated using physical methods, without chemical reactions. This is because they are not chemically bonded.
A compound is a substance that is made of more than one element. In a compound, elements are joined together by chemical bonds, which make it very hard to separate them.
When a compound is formed, the atoms of the elements join together in a fixed ratio. This means that each compound can be shown by a chemical formula.
For example, the formula of water is H₂O and the formula of carbon dioxide is CO2.
A mixture is made when two or more elements or compounds are together without being chemically joined.
The substances in a mixture are not in fixed amounts or ratios like they are in a compound, e.g. water has two hydrogen atoms and one oxygen atom. They can be in any combination, e.g. for a mixture of sand and water you can have any amount of sand with any amount of water.
Here are some examples:
Summary: