Displacement reactions
Displacement reactions in metals Iron displaces copper in copper sulfate In this example, we can say that the iron (Fe) displaced copper (Cu), as it has swapped positions with copper. Iron is now bonded to the sulfate (SO4) to form iron sulfate (FeSO4), while the metal in the solution has become copper, which is now on its own. The reactivity series with carbon For a displacement reaction to take place, the element on its own must be more reactive than the element that is in the compound. Let’s look at an example that wouldn’t work: Iron + Magnesium sulfate → As iron is more reactive than magnesium, it won’t displace magnesium, and therefore the reaction won’t occur.
Some chemicals react easily and quickly. They are called reactive chemicals.
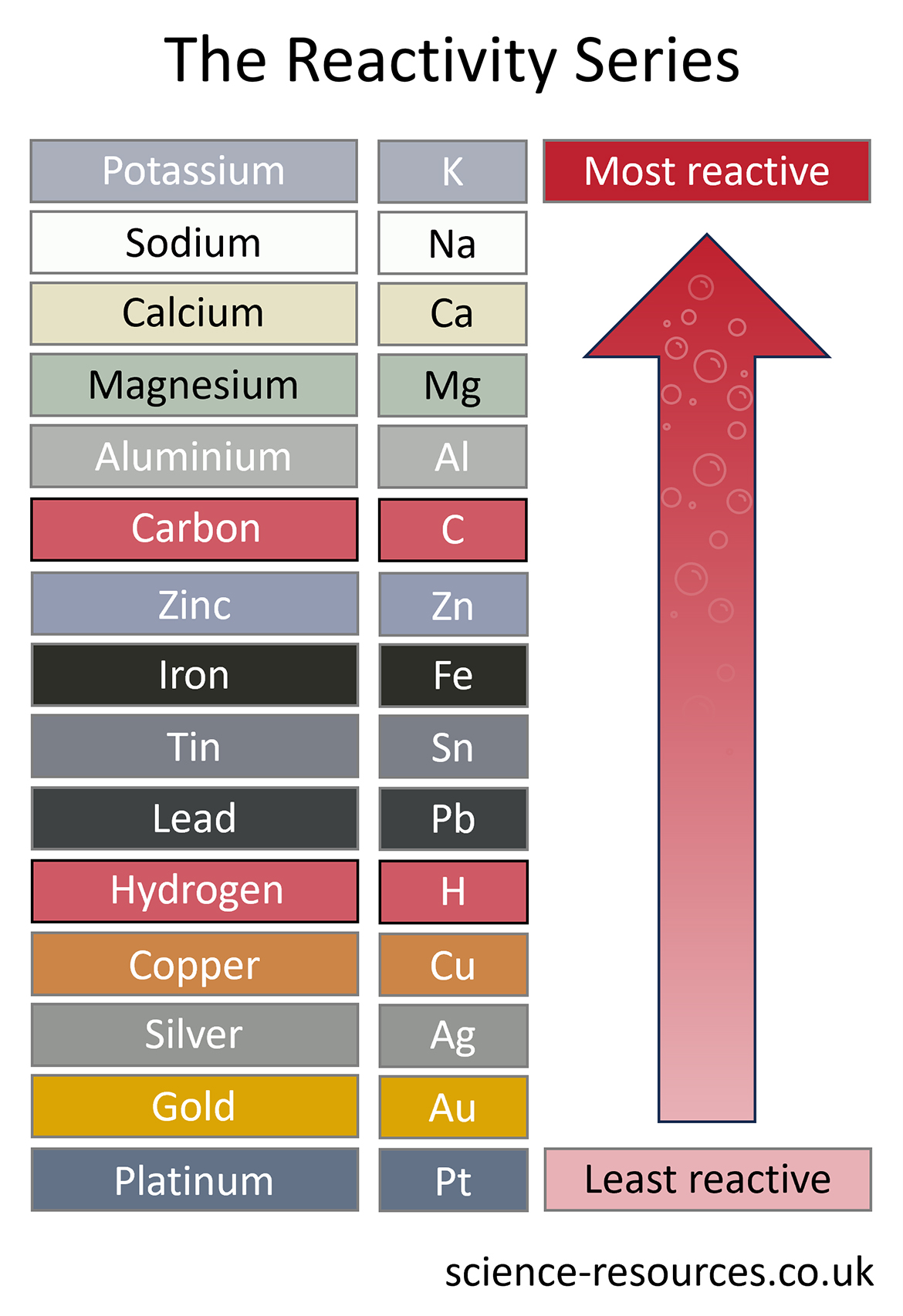
Metals have different levels of reactivity. We can make a list of metals from the most reactive to the least reactive. This list is called a reactivity series.
Metals that are not very reactive react slowly or not at all.
Displacement reactions happen when a metal and a compound of another metal are mixed. A compound is a substance that has two or more elements joined together. A metal that is more reactive can push out a metal that is less reactive from its compound. This is called displacing. The metal that is less reactive is left alone after the reaction. It is not joined to any other elements anymore. It is now a pure element.
Example
Below is an example of a displacement reaction between iron and copper sulfate:

The word equation for this reaction is:
Iron + Copper sulfate → Iron sulfate + Copper
These reactions take place due to differences in reactivity. Let’s take a look at the reactivity series:
In the previous example, iron is more reactive than copper, so it can take its place in a compound. When iron is put in a solution of copper sulfate (CuSO4), it displaces the copper and forms iron sulfate (FeSO4).
This means:
iron dissolves and makes a new solution with sulfate copper comes out of the solution and covers the iron as a solid
Fe + MgSO4 →
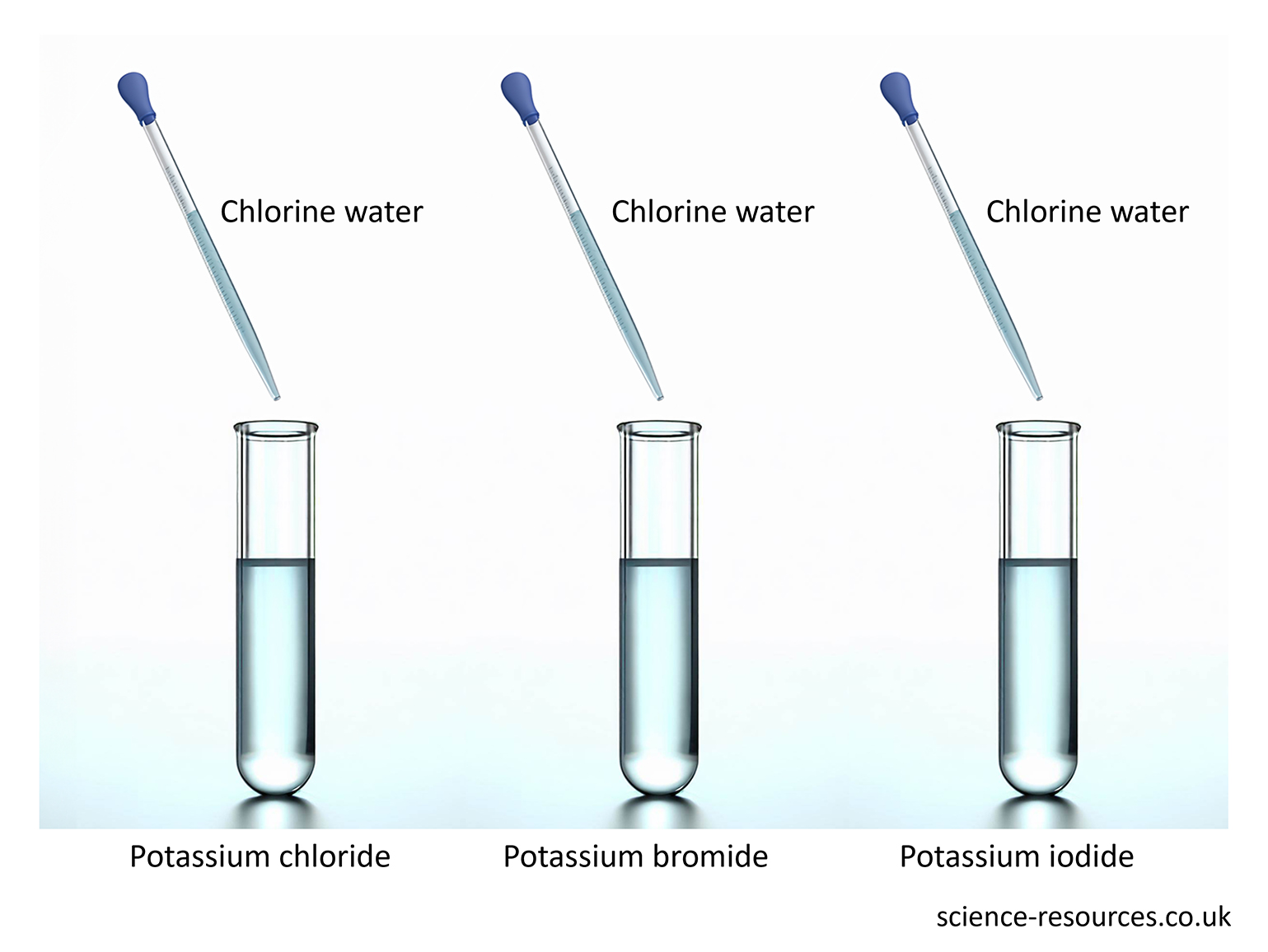
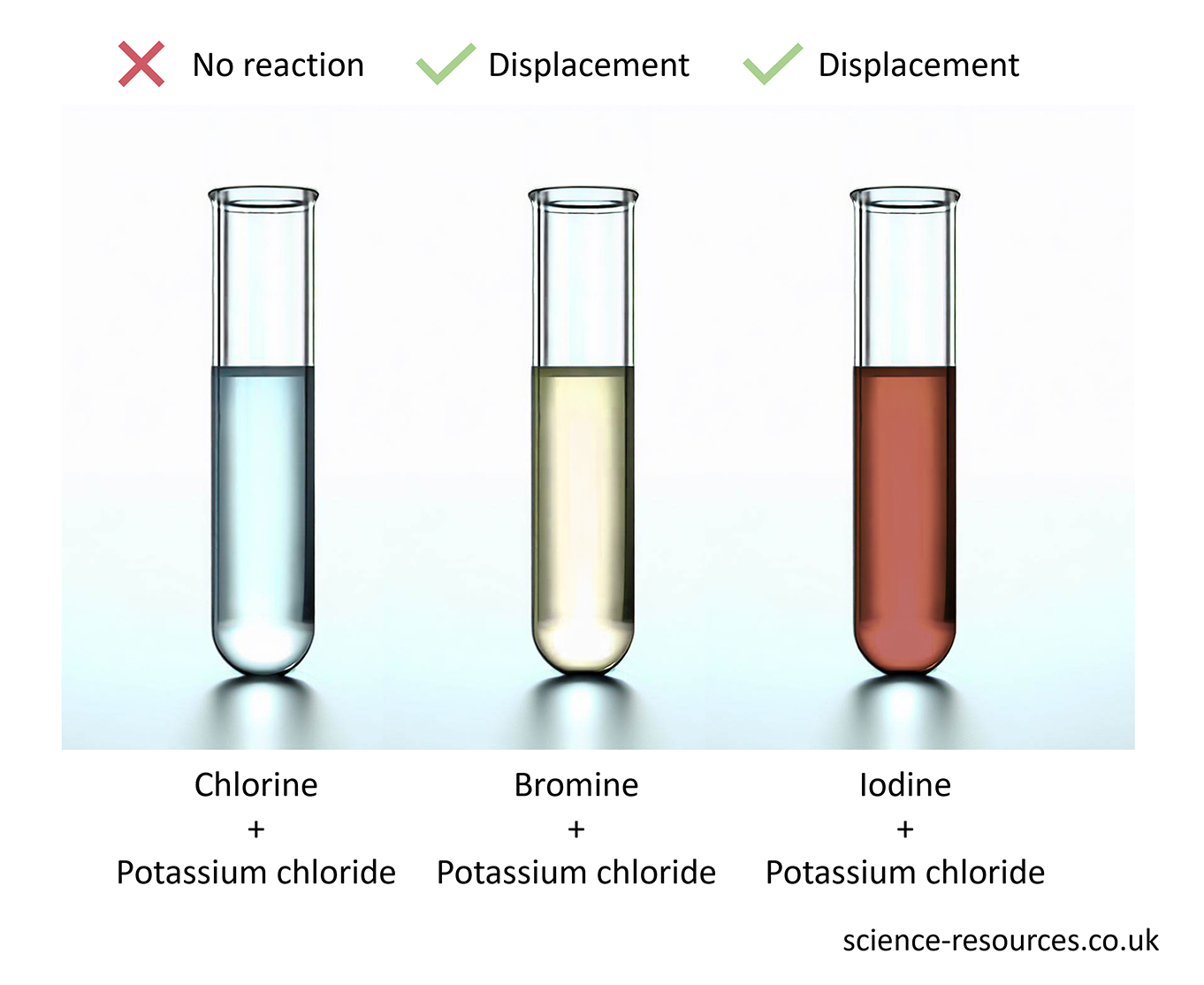
Extracting metals The reactivity series with carbon Displacement reaction in non-metals chlorine + sodium bromide → sodium chloride + bromine When bromine is mixed with sodium iodide, a new compound and a new element are made: bromine + sodium iodide → sodium bromide + iodine But when iodine is mixed with sodium chloride or sodium bromide, nothing happens. Iodine is not reactive enough to make those elements leave their compounds. The results of adding chlorine to these three solutions.
The method used to extract a metal from its ore depends upon its position in the reactivity series.
The simplest and cheapest way to extract a metal from its ore is to heat it with carbon. Carbon used to come from wood as charcoal, but now it comes from coal as coke. The ore is usually a metal oxide, and the reaction is like this:
metal oxide + carbon à metal + carbon dioxide
This method can be used to extract all the metals lower than carbon in the reactivity series. That is why carbon is often included in reactivity series diagrams.
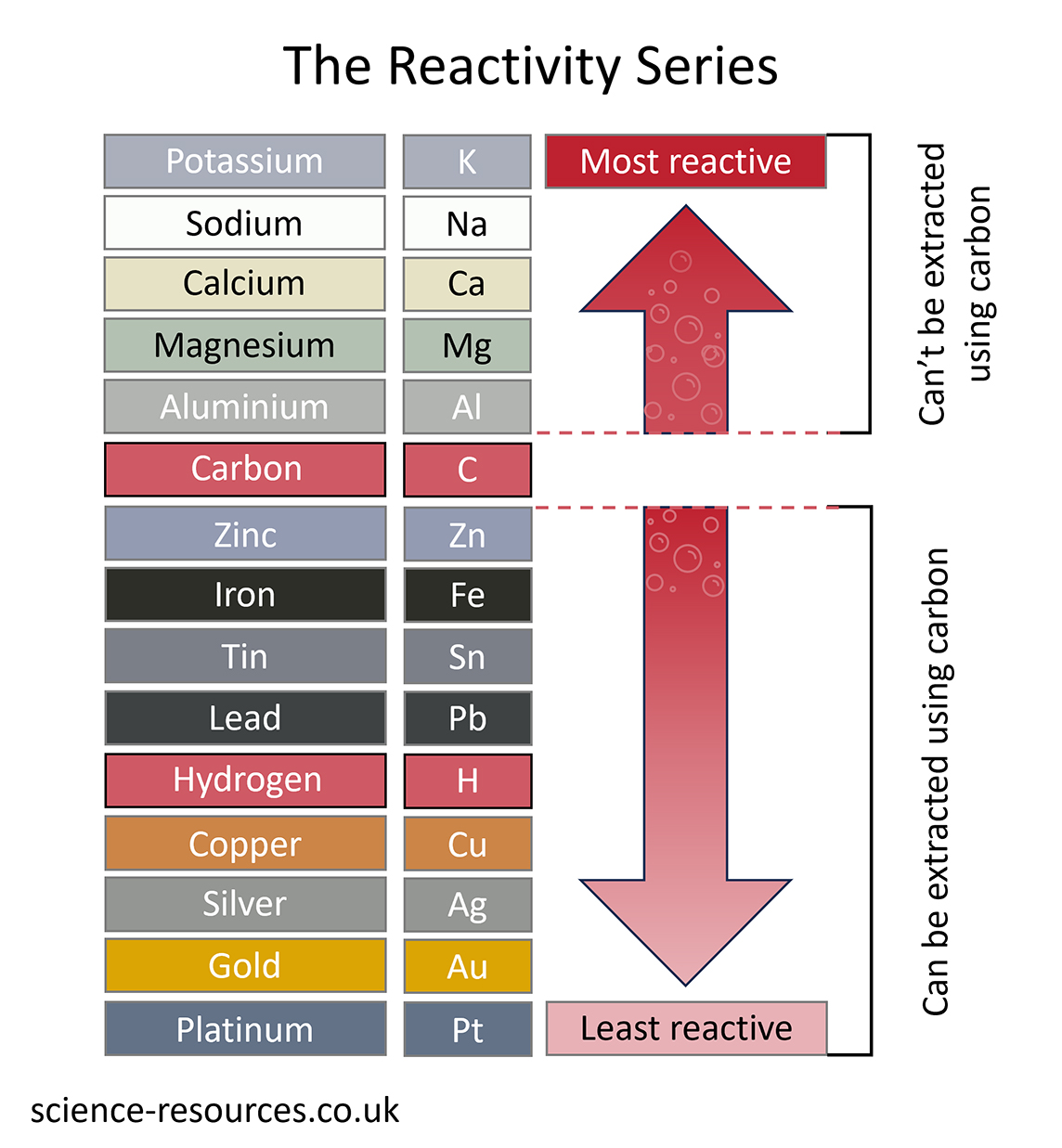
This method will not work for metals like magnesium, which are more reactive than carbon. Carbon cannot displace these metals.
Some non-metals react like metals in displacement reactions. Chlorine, bromine and iodine are examples of these non-metals. Chlorine is the most reactive and iodine is the least reactive. This means:
Chlorine can push out bromine and iodine from their compounds. Bromine can push out iodine but not chlorine. Iodine cannot push out chlorine or bromine. When chlorine is mixed with sodium bromide, a new compound and a new element are made:
Summary: