Exothermic and endothermic reactions
What are exothermic reactions?
Some chemical reactions release energy to the surroundings. This energy can be heat or light. The surroundings get warmer or brighter because of these reactions. These are called exothermic reactions. For example, handwarmers make heat when they react. They warm up your hands when you use them.
Not all exothermic reactions make heat. Some of them make light instead. For example, glowsticks make light when they react. They glow in the dark without getting hot.
What are endothermic reactions? Exothermic vs. endothermic reactions
Some chemical reactions take in energy from the surroundings. The surroundings lose heat energy and become cooler.
Plants use light energy from the sun to make their own food. This is an example of an endothermic reaction. The leaves of the plants absorb the light energy.
Some chemicals break down when they are heated. This is called thermal decomposition. These reactions are also endothermic because they need heat energy to happen.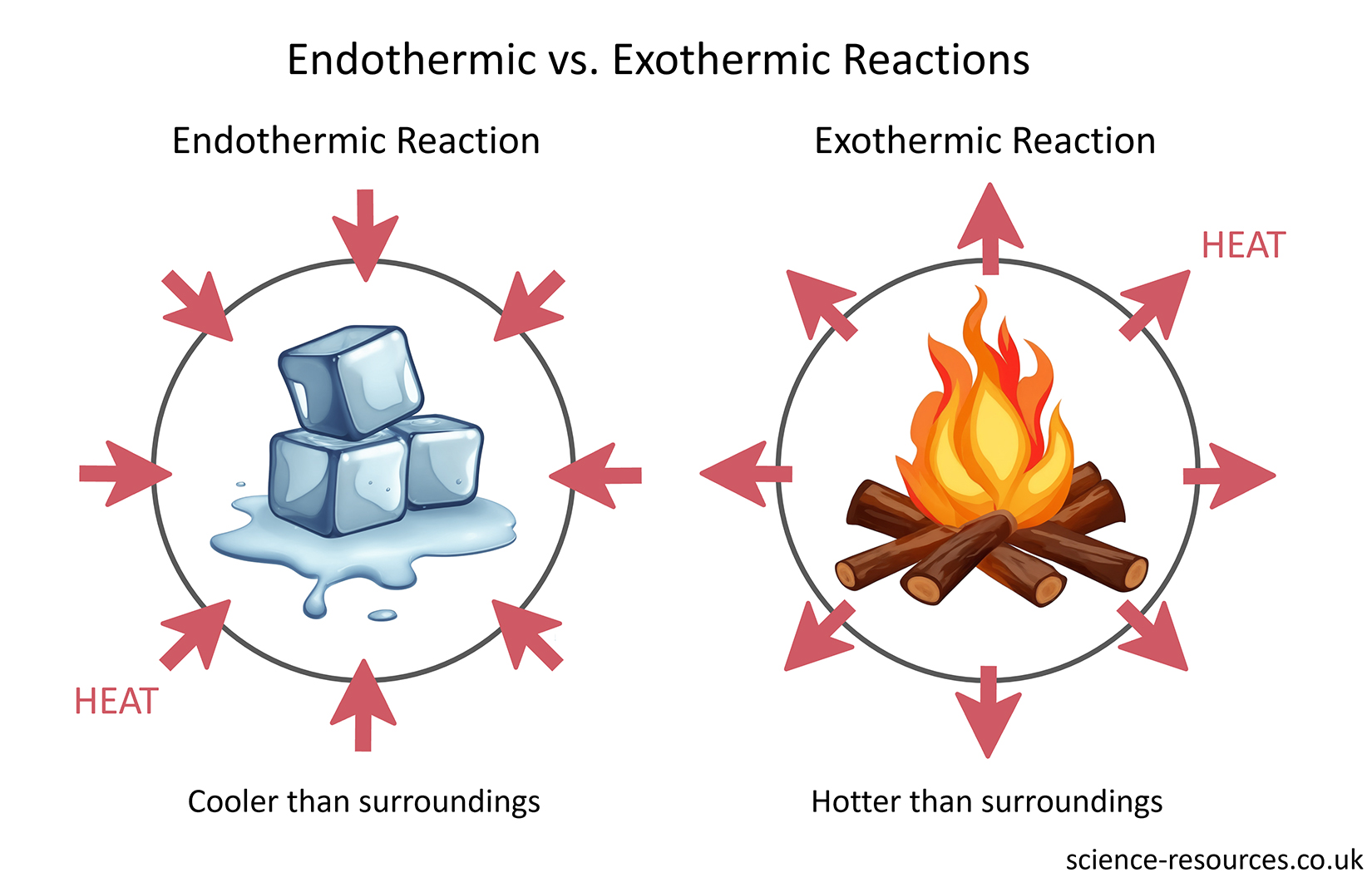
Summary: