Polymers
What are polymers? Natural polymers Synthetic polymers Synthetic polymers are produced by chemical reactions that connect many small molecules into long molecules. For example, polycarbonate is a synthetic polymer used to make the lenses for glasses. The above reaction is called addition polymerisation. One of the most common monomer is ethene. When these react with each other, a long chain molecule is produced, commonly called polythene There are many different building blocks that can be used to make plastics, so polymers can be designed for specific uses.
Formation of nylon
A polymer is a very long molecule. Polymers are made by joining together thousands of smaller, reactive molecules called monomers (the word 'mono' meaning one).
A polymer is like a long chain of daisies. Each daisy is a monomer, a small unit that repeats. When they are joined together, they make a polymer, a long molecule.
Some polymers are found in nature, such as cellulose, DNA and proteins. Cellulose is the main component of plant cell walls. It helps plants stay rigid and upright. The building blocks of cellulose are simple sugars. The simple sugars link together to form a long chain molecule, which is the polymer cellulose.
Natural polymer (Natural monomer)
Look at the animation to see how ethene molecules combine to form long molecules of the synthetic polymer called polyethene.
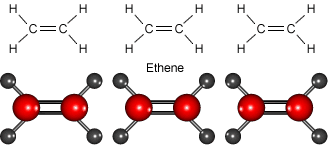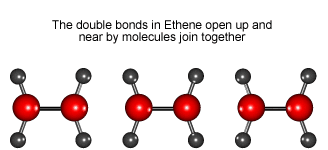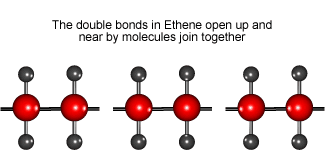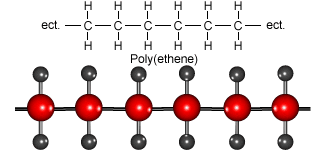
Ethene molecules join together to make polyethene.
Plastics are the common name for synthetic polymers. Plastics are everywhere. They are one of our most useful materials.
Synthetic polymers often share these properties:
Properties and uses of polymers Advantages: Disadvantages: Different polymers show these properties to different extent. Some polymers can be remoulded as many times as desired after first softening them by heating. These are called thermoplastic polymers. They are sometimes called thermosoftening plastics. Thermoplastic In the thermoplastics the polymer chains are totally free to slide past each other, therefore it is easy to change the shape. Thermoset In thermosetting polymers the chains are cross-linked. Instead of each chain being separate, adjacent chains are linked together. This makes it difficult for polymer chains to move past each other, hence the polymer is hard and rigid. Even when it is heated, the chains are still unable to move, so the polymer does not melt. Rubber is natural polymer produced by rubber trees. It is a runny, sticky liquid called latex. In its raw form it far too soft and sticky to be of much use. But if sulphur is added to rubber, it makes cross-links between the polymer chains, and the rubber gets harder. This is called vulcanising the rubber. The higher the content of sulphur, the more cross links are formed and the harder it gets. For example, eraser is soft rubber and has far less sulphur content in it then a car tire, which needs to be very hard. Polymers and their uses Monomer: Properties: Very cheap, strong and easily moulded Polypropene Polystyrene Properties: Cheap, easily moulded and can be expanded into foam Polychloroethene (PVC: Polyvinyl chloride) Properties: Cheap, strong, flexible yet strong, etc. Nylon (made from 2 different monomers) 1,6 diaminohexane Phenolic resins: Bakelite (made from 2 different monomers) Phenol + Aldehyde Perspex (acrylic) PTFE The problem with polymers
Polymers have properties which make them very suitable for all sorts of objects.
Other polymers soften and can be moulded the first time they are heated, but can't be resoftened and remoulded. These are called thermosetting polymers. If you heat them strongly enough, they eventually break down and char. They are hard and rigid.
These different properties can be explained if you look at the arrangement of the polymer chains.
The diagrams below show the polymer chains in thermoplastic and thermoset polymers.

separate polymer chains.

For example, polythene is very cheap and is easily moulded into strong, flexible containers. However, it pollutes the environment as it does not rot.
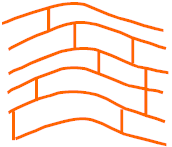
Chains fixed together by strong bonds

This electrical plug is made from a thermosetting plastic which does not melt when it gets hot.

Rubber for car tires
Polythene
![]()
Use: Carrier bags, moulded for containers, buckets, pop bottles, clingfilm etc.
Monomer:
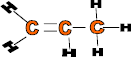
Properties: Form strong fibres and has high elasticity, flexible, light and can be dyed
Use: Car parts such as bumpers, battery cases, plastic chairs, ropes, fishing nets and carpets.
Monomer:
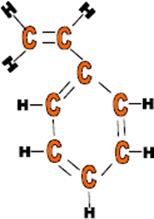
Use: Plastic models, foam packaging, plastic cups, radio outer cases and when expanded, as insulation.
Monomer:
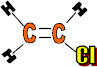
Use: Pipes, gutters, window frames, electrical insulation of cables, floor tiles, rain coats, seat covers, records and wall paper.
Monomer:
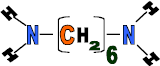
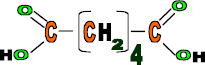
Hexanedioic acid
Properties: Cheap, light, flexible, strong fibre, Can easily catch fire etc.
Use: Ladies tights, ropes, bristle for brushes, carpets and clothing.
Monomer:
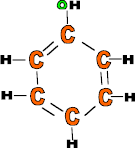
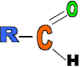
Properties: Cheap, very strong but brittle, rigid, etc.
Use: Electrical plugs, switches and saucepan handles.
Monomer:
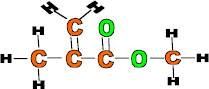
Properties: Strong, clear and easily moulded
Use: Car light reflectors, safety glasses, contact lenses, traffic signs and false teeth.
Monomer:
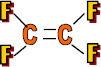
Properties: Very slippery, non-stick & hard
Use: Non-stick surfaces, Teflon (pans, skis) and gaskets
Polymers are usually not chemically reactive. This is a useful property because it means that plastic bottles will not react with their contents. For example, a fruit juice drink has lots of different acids. It is important that the plastic in a drink bottle does not interact with them.
Unfortunately, this low reactivity also makes polymers hard to dispose of. When they are thrown away, they do not decompose very fast and so stay in the environment for a long time. Plastic pollution is a big environmental problem.
Summary: