Solids, liquids and gases
Solids A particle model of a solid (Note: Particles are all touching) Liquids A particle model of a liquid (Note: Particles are close together) A particle model of a gas (Note: Particles are far apart)
Many materials are solid, such as paper, bricks, wood, metal, and ice.
The particles in solids are packed tightly together, so they usually cannot be squeezed or compressed. The particles are held together by strong forces of attraction that keep them in a fixed position.
The particles in solids have a regular pattern of arrangement. The particles in solids only vibrate around their fixed position. This makes solids have a fixed shape and prevents them from flowing like liquids.
The particles in solids vibrate faster when they get hotter. This means that solids get bigger when they are heated.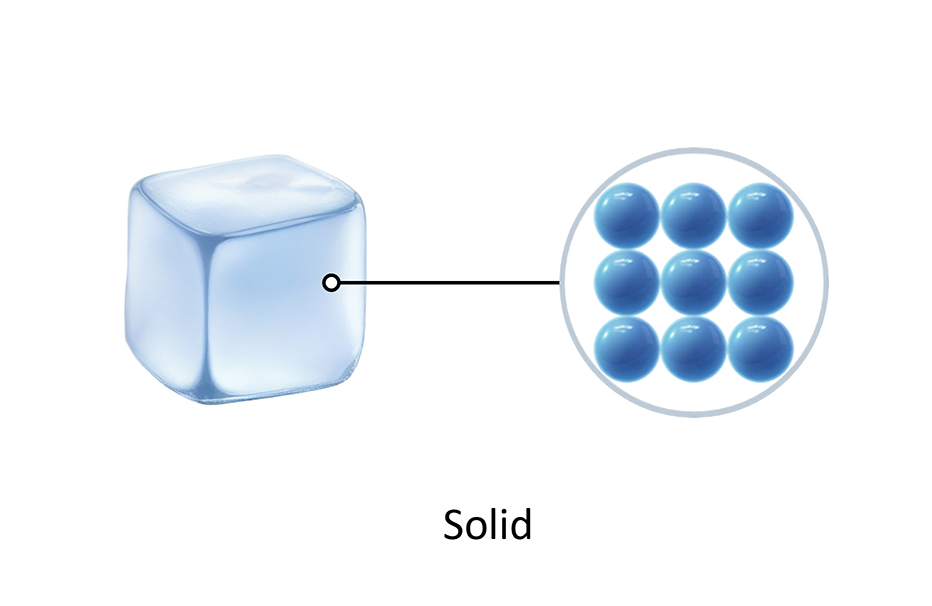
Water, oil, fruit juice, and many others are examples of liquids.
The particles in liquids are randomly arranged, and are close to each other, touching many other particles. There are some spaces, but liquids usually cannot be squeezed or squashed.
The particles of a liquid have enough energy to overcome some of the forces of attraction between them. So, particles in liquids can move freely and slide past each other, making liquids flow and be poured. 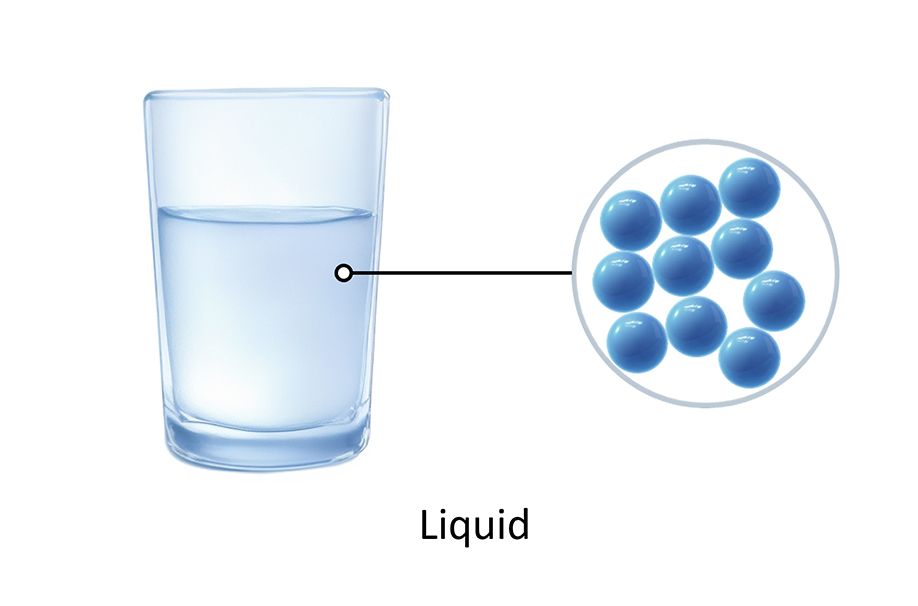
Gases
The air we breathe and the helium that fills balloons are examples of gases.
The particles in gases are far apart and randomly placed, so they can be easily squashed or compressed.
The particles in a gas have enough energy to break free from the forces of attraction between them, so they can move in any direction. They move fast in straight lines, bumping into each other and the walls of their container.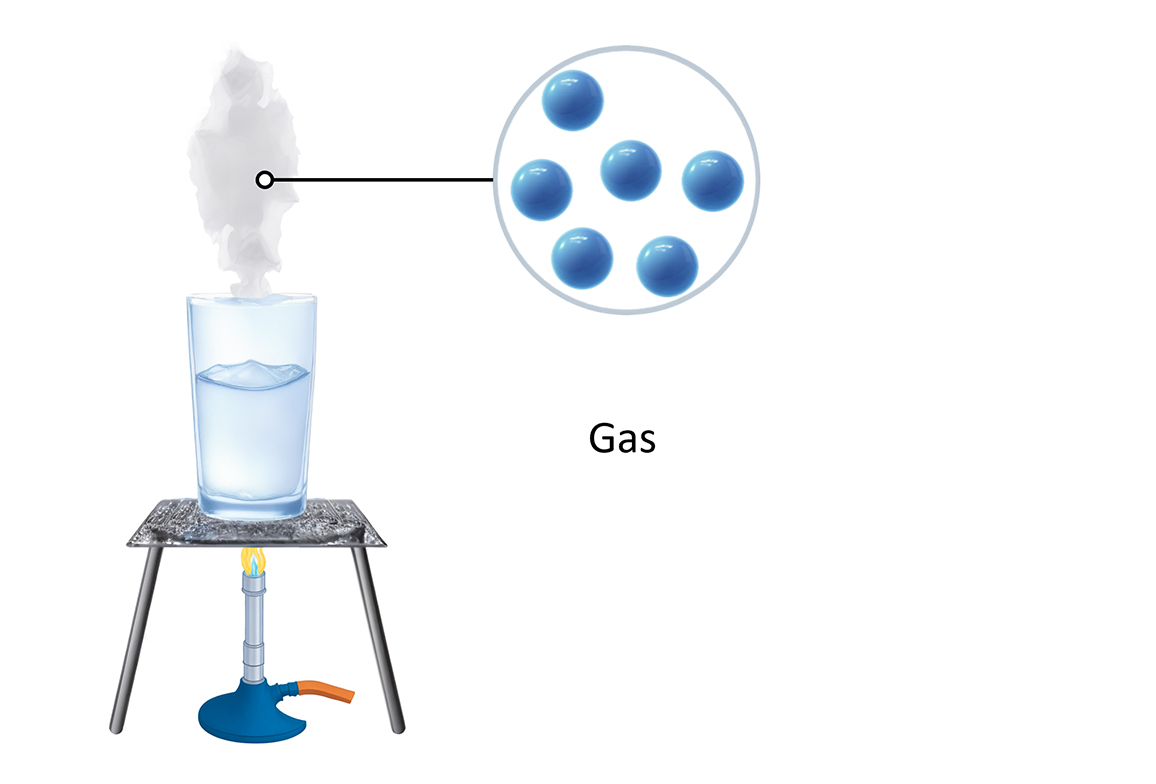
What are particles? What are models?
Particles are the tiny bits of matter that make up almost everything, except for things like light and X-rays that are electromagnetic waves.
A particle is a single piece of matter from an element or a compound, that is too small to see, even with the strongest microscope in a school science lab. Particles can be single atoms, groups of atoms called molecules, or atoms with electric charges called ions.
Scientists use models to help explain scientific ideas. The particle model is the name for the pictures used to show solids, liquids and gases.
In the model, the particles are drawn as circles or balls. But the particles in ice, liquid water and steam are the same because they are all water, just in different forms of matter.
Models are useful to show things that are too small to see, like atoms or cells. 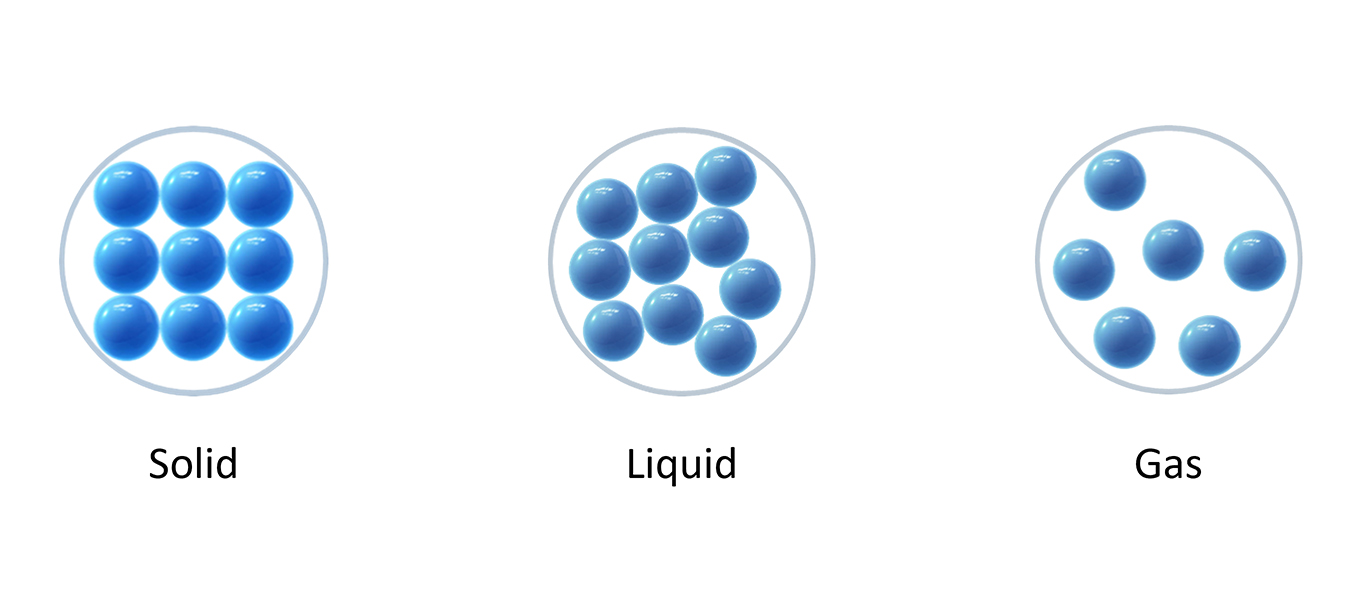
Summary: