Modern periodic table
The periodic table Periodic table of elements The table has one or two letters in each square, which are the element symbols. This makes the table less crowded and easier to find the elements.
In the modern periodic table:
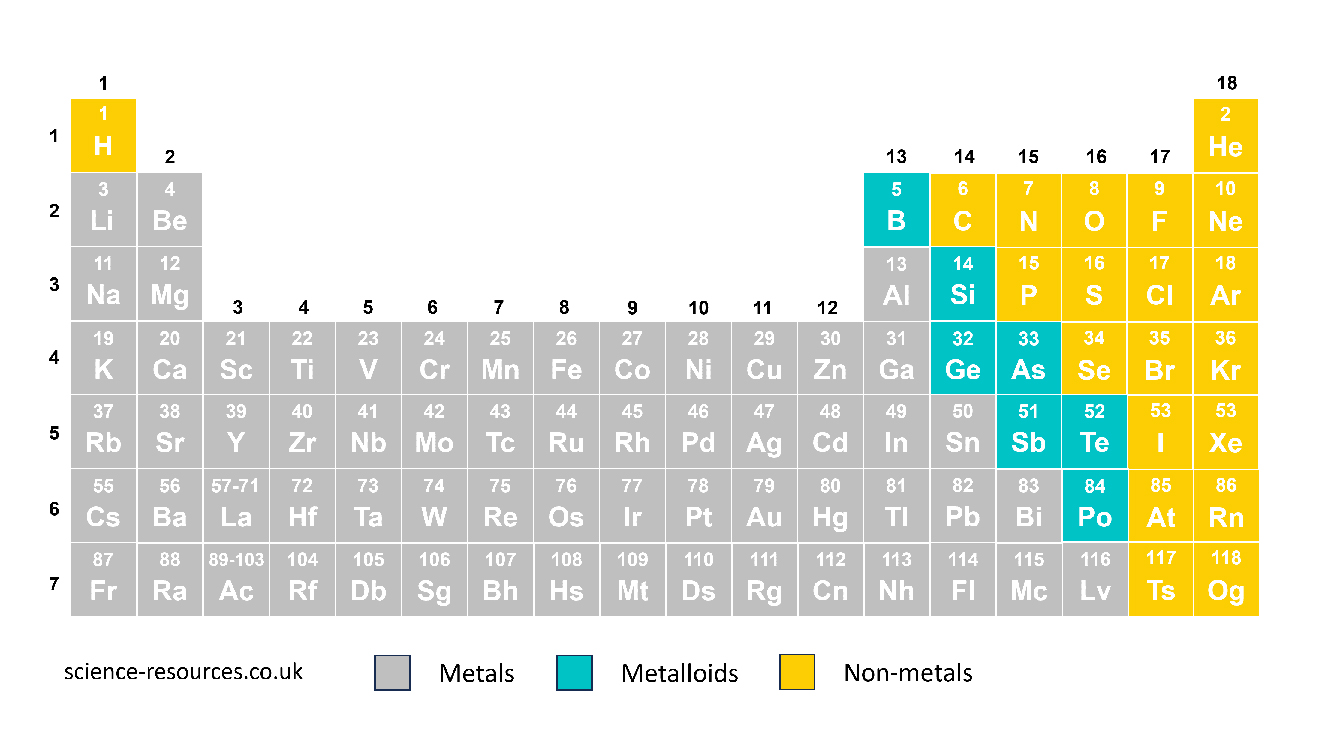
The element symbol always begins with a big letter. If there is another letter, it will be a small letter, for example, He for helium. Some element symbols are easy. For example, O for oxygen. Others are not so easy. For example, Fe for iron, from the Latin word ferrum for iron.
The arrangement of the periodic table Alternative version of the periodic table of elements You can read the periodic table from left to right along the horizontal rows or from top to bottom along the vertical columns. Elements in the same group have similar chemical and physical properties. For example, group 1 has metals that react easily, while group 0 has gases that do not react at all.
The left and middle parts of the periodic table have metals, and the right part has non-metals. A line that zig-zags separates them. Hydrogen (H) is a non-metal. In some versions of the periodic table, hydrogen is shown by itself because it is very different from other elements.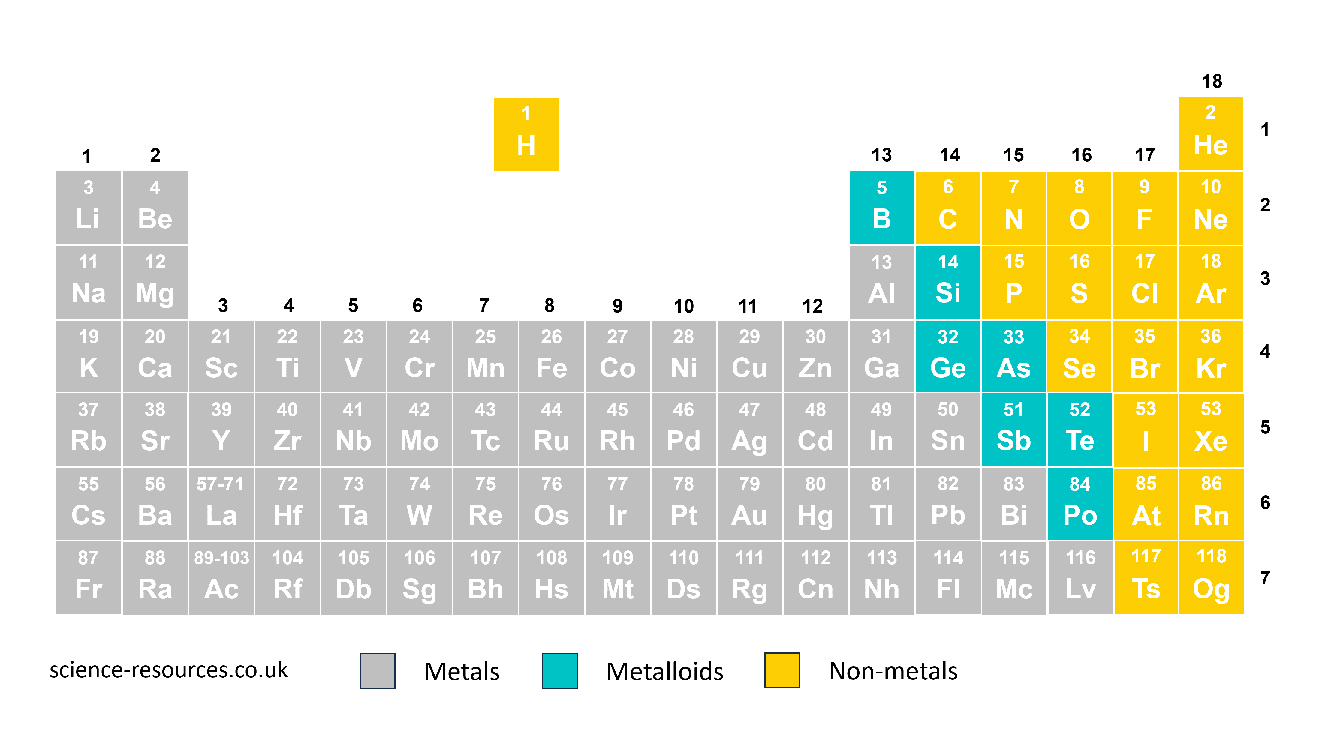
The horizontal rows are called periods. They go from one side of the periodic table to the other, even if there is a space. For example, the fourth period has Potassium (K) to Krypton (Kr) elements. The vertical columns are called groups and have numbers. For example, group 13 has Boron (B) elements down to Nihonium (Nh).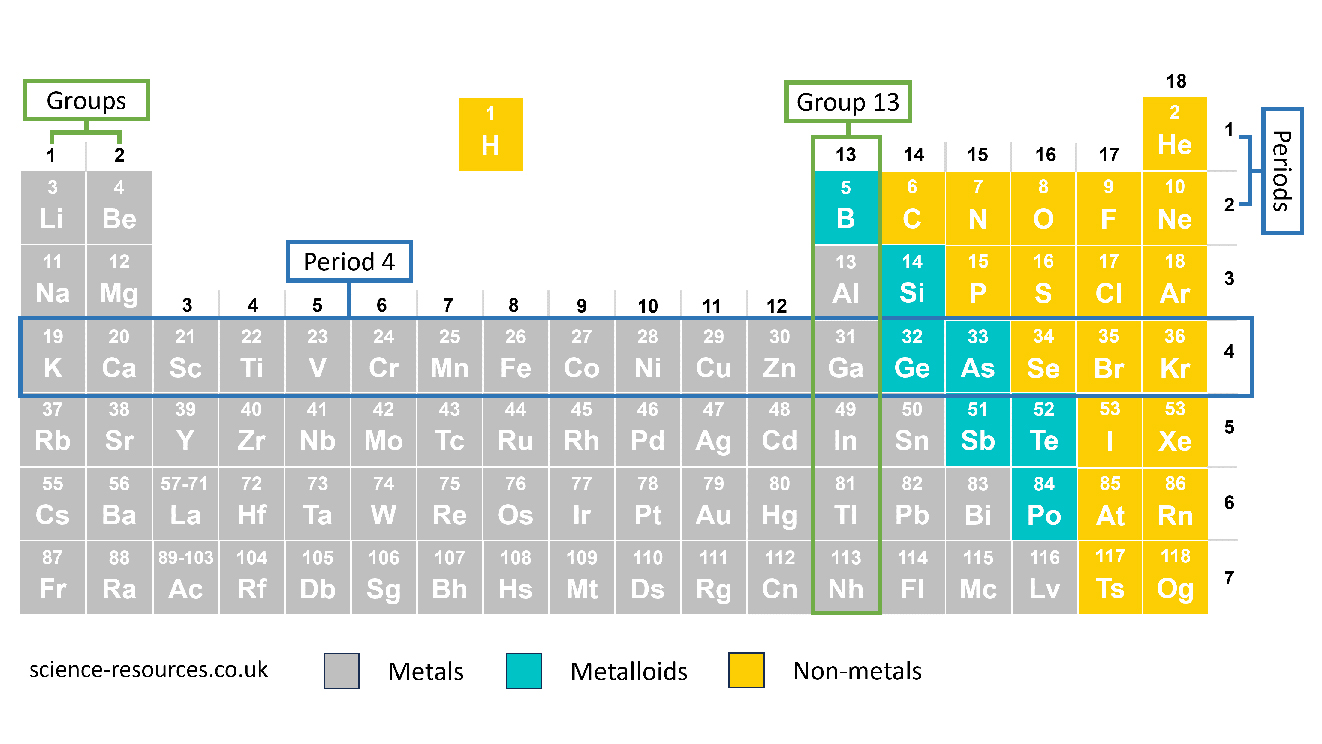
Different periodic tables may look different. They may have different labels for the groups or numbers for the elements. But the elements always stay in the same place.
The way the periodic table is arranged helps us to predict how elements will behave based on their position. This makes the periodic table very useful for scientists.
Summary: