Physical and chemical properties
Properties Periodic table of elements Physical properties This picture shows how water changes its physical state when it is heated or cooled. Water changing state
A property tells you how a substance acts. There are 2 kinds of properties:
Chemical properties - these are changes that happen when substances react with each other and make new substances.
Physical properties - these are changes that only affect the physical state of a substance. For example, changing from solid to liquid.
There are many metals in the periodic table, almost 100. It is important to know the properties of a substance to choose the right material for a use. 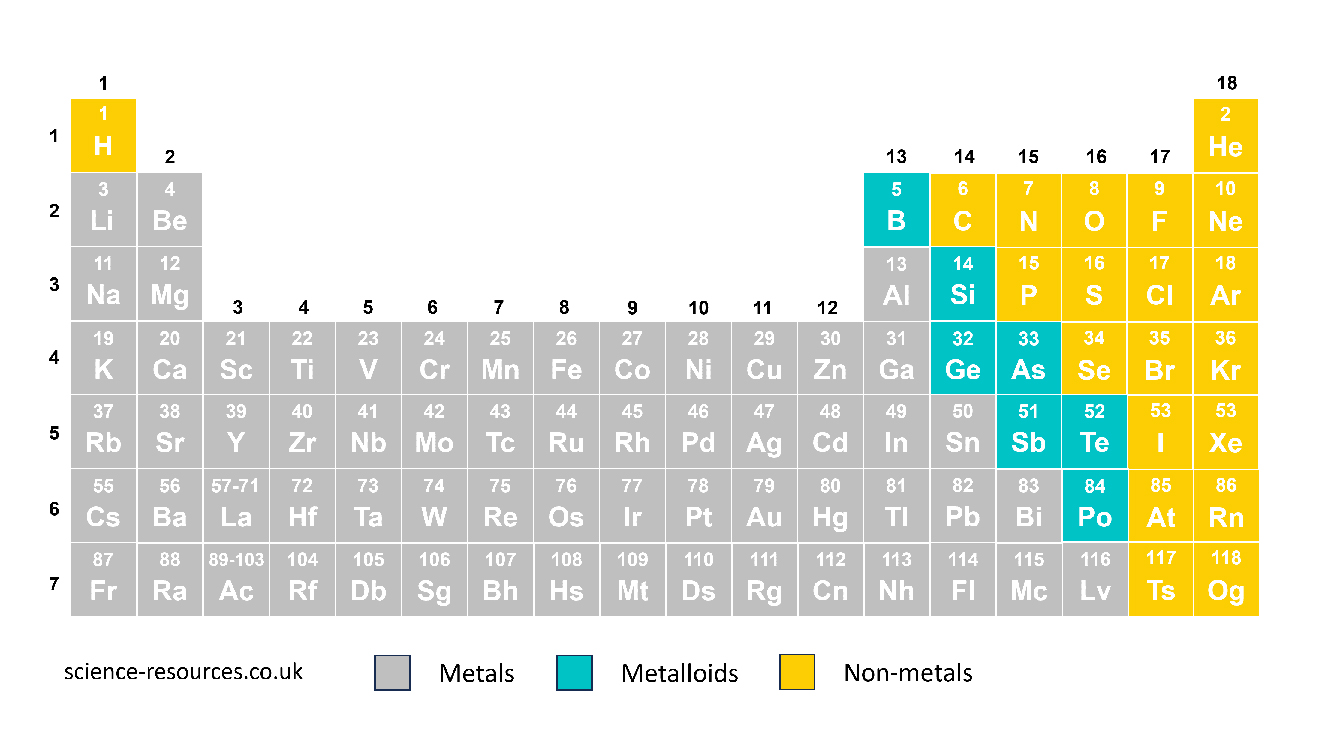
Physical properties are things that you can see and measure easily in an experiment. Some examples are:
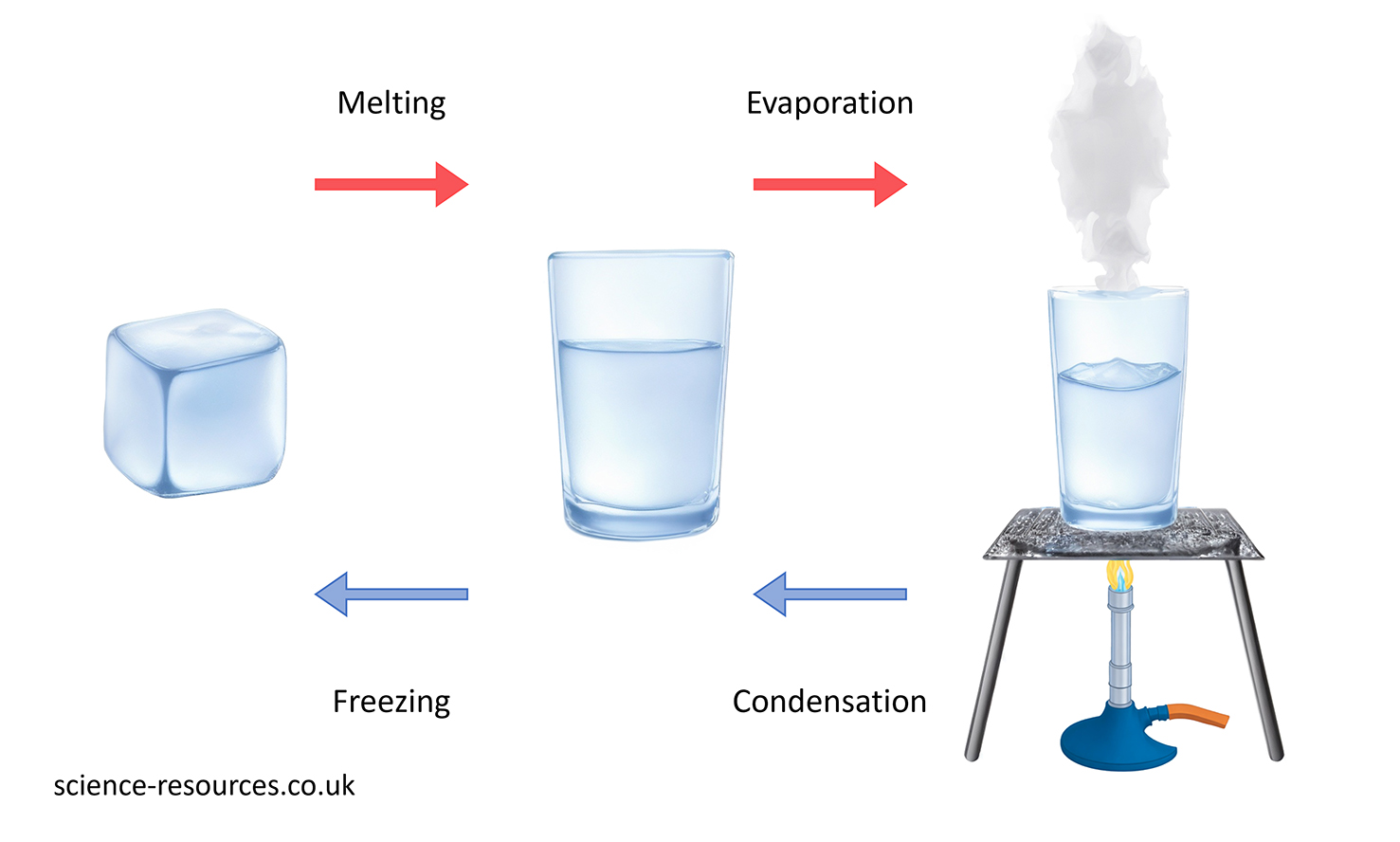
Chemical properties Some ways to measure chemical properties include seeing how elements react with air. For example, the elements in Group 1 react with air that has water in it. They are kept in oil to prevent them from touching air and water. When potassium touches moist air, it makes potassium oxide. This covers the metal with a layer.
Chemical changes are when substances react with each other and make new substances. You cannot tell the chemical properties of a substance just by looking at it, touching it or measuring it easily.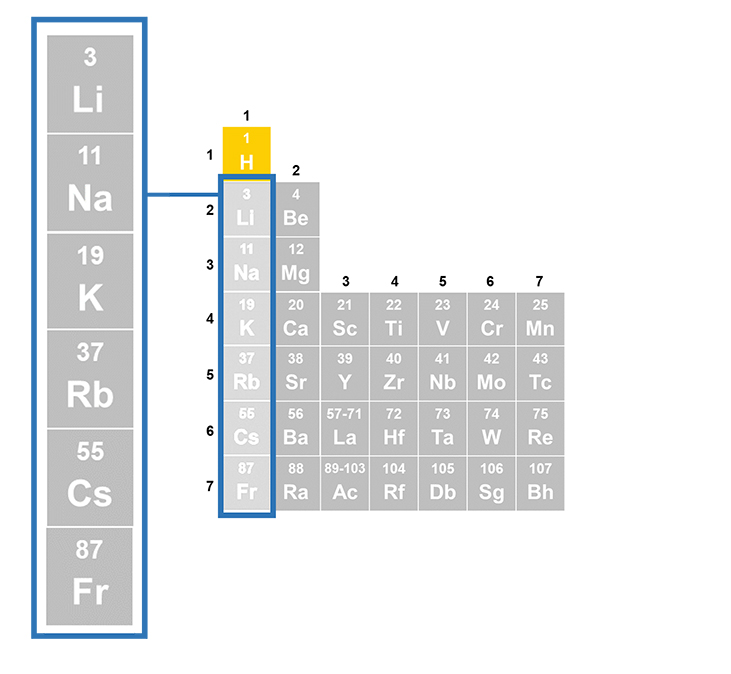
Summary: