Evaporation
What is evaporation? Using evaporation to separate a solution A solution is placed in an evaporating dish and heated with a Bunsen burner.
Evaporation is when a liquid changes into a gas slowly, without reaching its boiling point.
Evaporation can happen at any temperature, but boiling only happens at a specific temperature for each liquid.
Evaporation can be used as a separation method to separate components of a mixture with a dissolved solid in a liquid. The liquid is evaporated, meaning it is converted from its liquid state to gaseous state. This often requires heat. Once the liquid is completely evaporated, the solid is all that is left behind.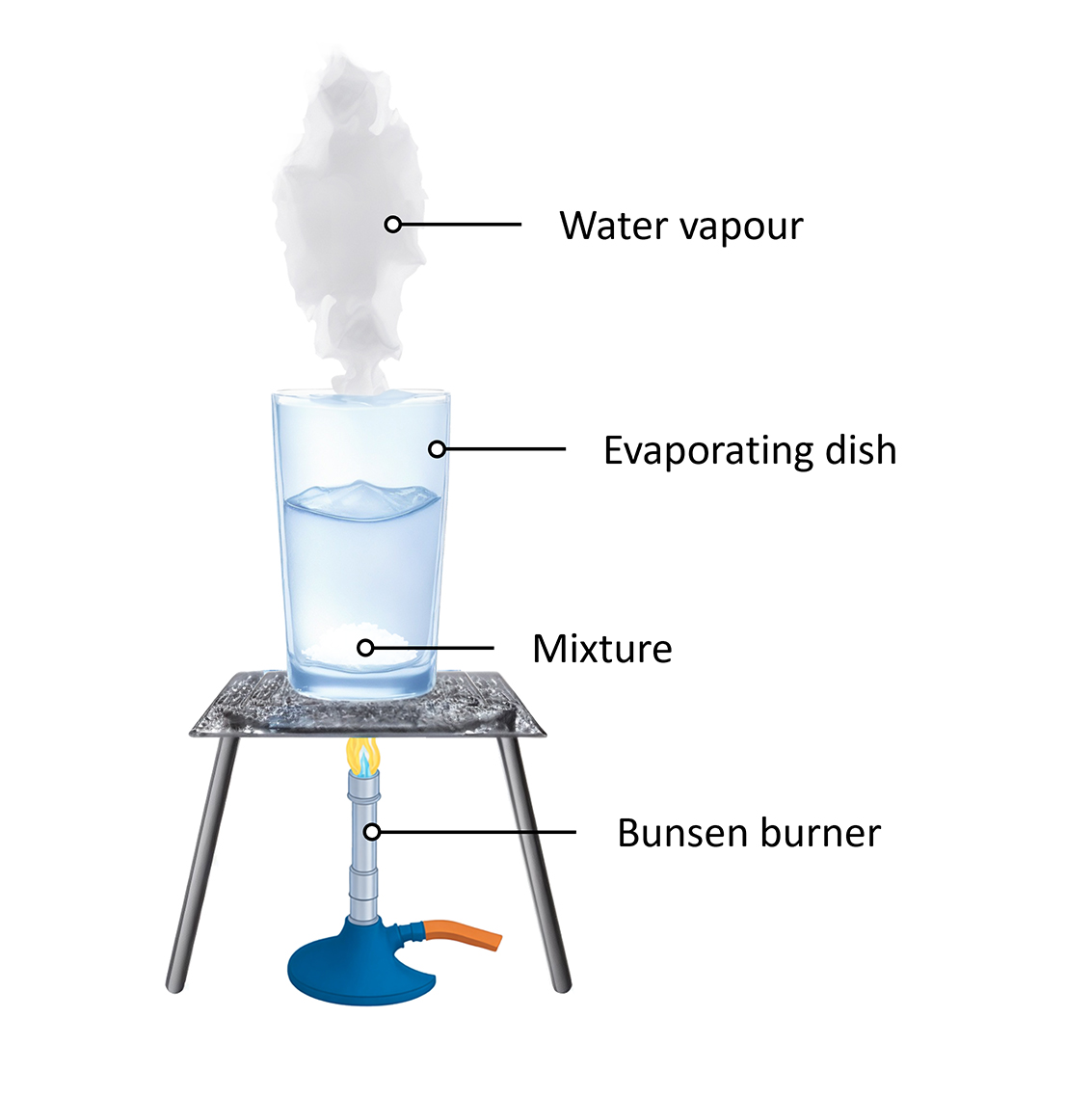
Crystallisation
Crystallisation is a way of separating a solid solute from a solution by letting the solvent (water) evaporate slowly over time.
Crystallisation makes the solute form bigger crystals, because the slower the water evaporates, the more time the solute has to arrange itself into a regular pattern.
These are the steps for crystallisation:
Summary: