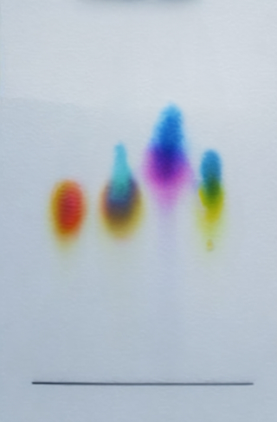Separating mixtures
Different methods can be used to separate mixtures, such as filtration, crystallisation, distillation or chromatography. The method that is chosen depends on the kind of mixture. Sieving Filtration
Separation
Mixtures can be easily split up. Methods like sifting, filtering, chromatography and evaporating can be used.
A mixture that has solid particles of different sizes, for example sand and gravel, can be separated by sieving.
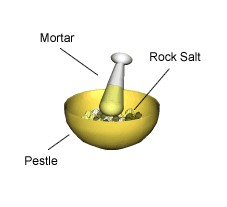
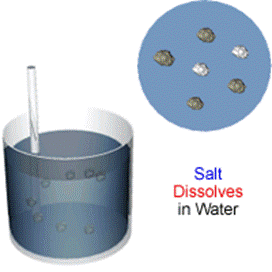
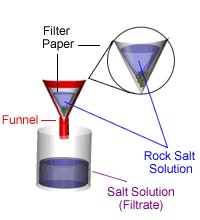
Filtration is a way of separating a solid that does not dissolve in a liquid. When a mixture of rock salt and water is filtered:
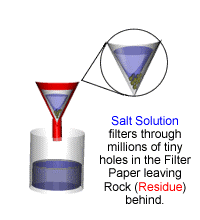
The rock salt remains in the filter paper (it is called the residue) the water goes through the filter paper (it is called the filtrate).
Evaporation Simple distillation Every pure substance has a specific temperature at which it changes from solid to liquid or from liquid to gas. One way to test how pure the separated liquid is to measure its temperature when it boils. For example, pure water boils at 100°C. If it has any solids mixed with it, like salt, its temperature when it boils will be more than 100°C. That is why salt is often added to water when cooking. The water boils at a higher temperature and so the food cooks faster.
Evaporation is a way of separating a solid that dissolves in a liquid. For example, copper sulfate can dissolve in water – its crystals mix with water to form copper sulfate solution.
During evaporation, the water changes into a gas and leaves behind solid copper sulfate crystals.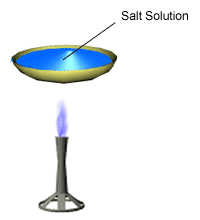
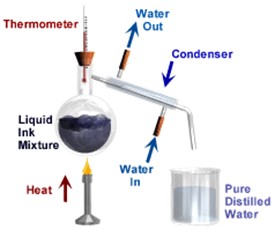
Simple distillation is a way of separating the liquid from a solution. For example, water can be separated from salt solution by simple distillation. This method works because water has a much lower temperature at which it boils than salt. When the solution is heated, the water changes into a gas. It is then cooled and changed back into a liquid in a different container. The salt does not change into a gas and so it remains in the original container.
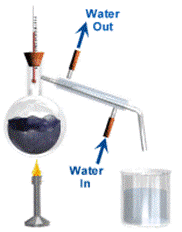
Fractional distillation Chromatography A chromatogram with different samples of ink.
Fractional distillation is a way of separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different temperatures at which they boil.
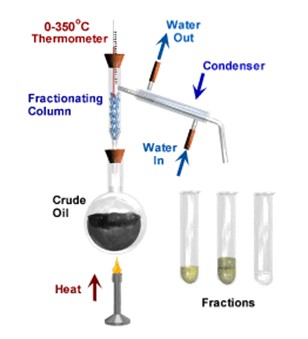
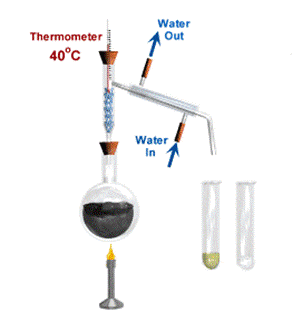
When the mixture is heated, the liquid that boils at the lowest temperature boils first. The gas changes back into a liquid in the condenser before the other liquid boils. The long fractionating column makes sure that the second liquid does not get into the condenser until most of the first one has been collected.
Paper chromatography is a way of separating dissolved substances from each other. It is often used when the dissolved substances have different colours, such as inks, food colourings and plant dyes. For example, ink is a mixture of colours dissolved in a liquid of water or oil.
It works because some of the colours mix with the liquid better than others, so they move further up the paper.