Extracting metals
Metals and their uses Unreactive metals Metals and their compounds Ores
Metals are very useful materials that we use every day. Many industries need metals for different purposes.
Metals come from rocks in the Earth’s crust. We need to process the rocks to get the pure metal. This is called extraction and it can have several steps, including chemical reactions.
Silver and gold are the most unreactive metals. They are not in compounds with other elements in the rocks. They are elements by themselves.
For example, gold (element symbol Au) is an unreactive metal and it is an element in rocks.
Most metals do not occur in their natural state. They are often found as compounds. These compounds are called minerals.
An ore is a rock which contains a high concentration of a metal compound, high enough for it to be worthwhile trying to get the metal from it. Getting a metal from its ore is called extraction.
Extracting metals The reactivity series with carbon and hydrogen Extracting iron Extracting aluminium .
The method used to extract a metal from its ore depends upon its position in the reactivity series.
The simplest and cheapest way to extract a metal from its ore is to heat it with carbon. Carbon used to come from wood as charcoal, but now it comes from coal as coke. The ore is usually a metal oxide, and the reaction is like this:
metal oxide + carbon --> metal + carbon dioxide
This method can be used to extract all the metals lower than carbon in the reactivity series. That is why carbon is often included in reactivity series diagrams.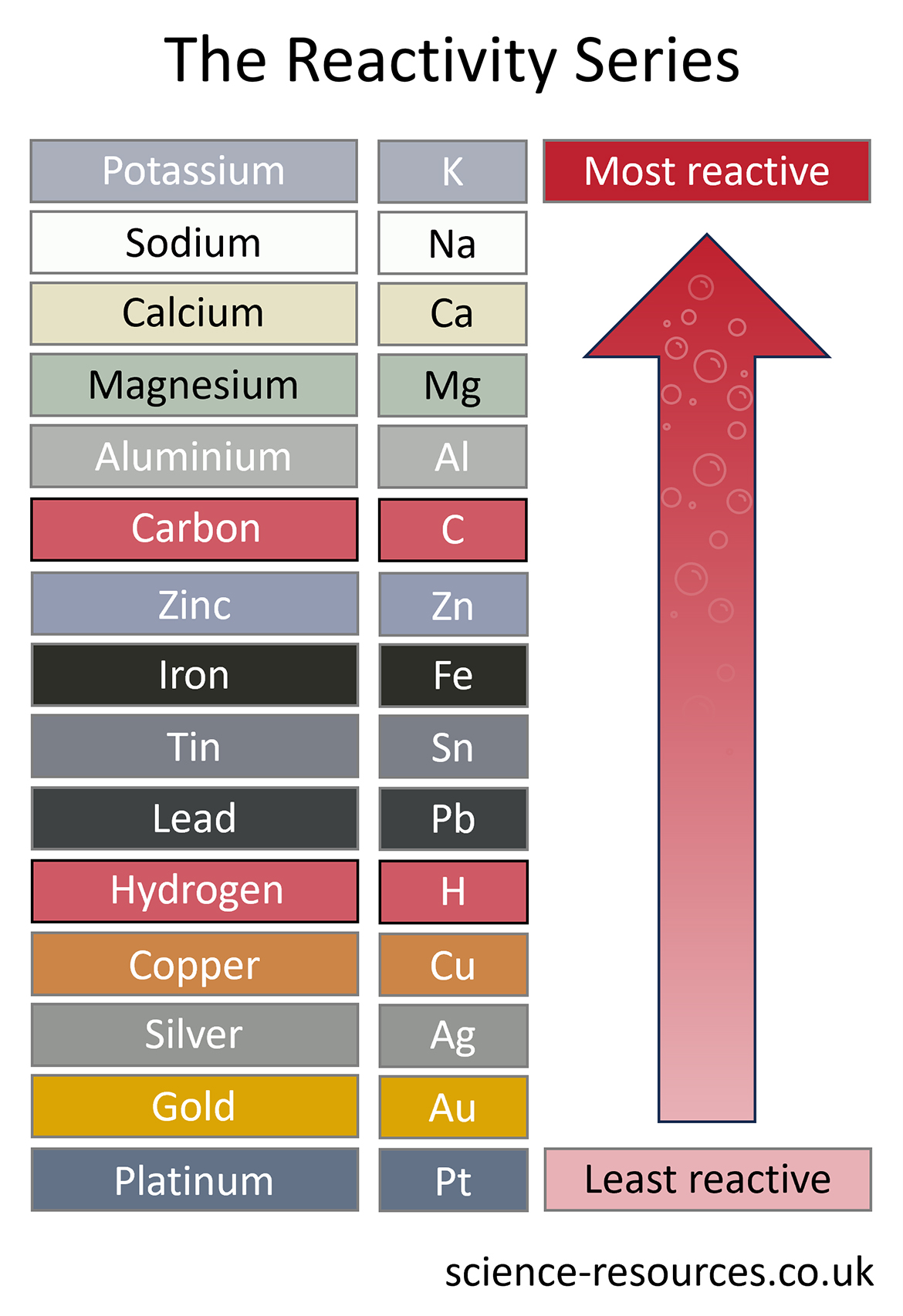
Iron is the most commonly used metal. It comes from an ore called haematite, which is mainly iron oxide. The iron is extracted by heating the ore with coke in a big furnace.
These reactions can be described using either word or symbol chemical equations. For example, the word equation for the reduction of iron oxide with carbon is:
carbon + iron oxide --> iron + carbon dioxide
Most iron is then made into steel, which is stronger, by adding a little bit of other elements to it.
Aluminium is the second most commonly used metals. It is used for things like planes, power lines and drinks cans. But aluminium is more reactive than carbon. So, we cannot extract it from its ore (bauxite, mostly aluminium oxide) by heating it with carbon. We could use magnesium or potassium to extract it, but they are very costly and hard to extract themselves. We extract aluminium by passing electricity through melted aluminium oxide at very high temperatures. This costs more than extracting iron because it needs a lot of power.
Summary: