Energy stores
What is energy? Energy stores Some examples of common energy stores
Energy is a quantity that is conserved – it cannot be created or destroyed; it can only be stored or transferred.
Common energy stores include:
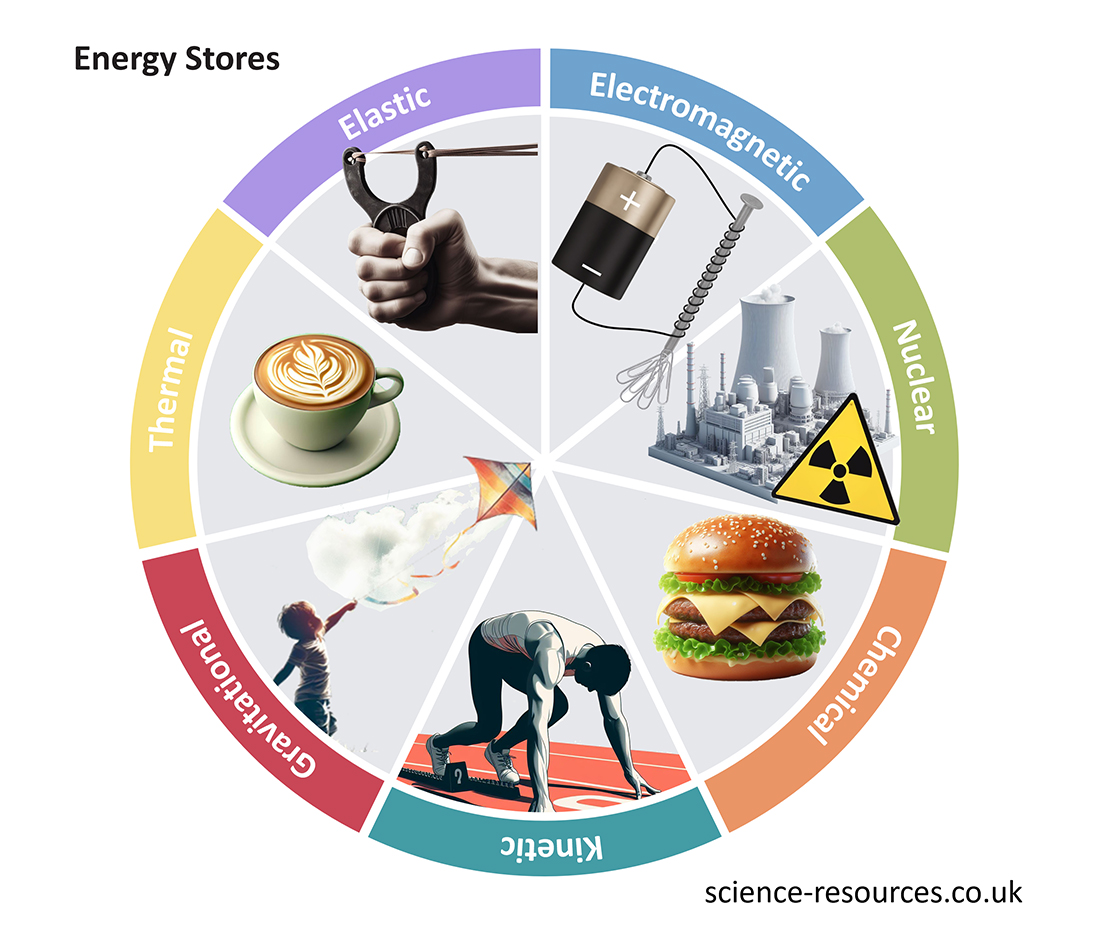
Thermal energy Gravitational potential energy store Kinetic energy store Elastic potential energy Chemical energy Conservation of energy Energy dissipation (energy waste)
A hot object has more energy in its thermal energy store than a cold object. The temperature of the object determines the amount of energy in the thermal energy store.
The amount of energy in the gravitational potential energy store depends on the height of the object. For example, a box has more energy in its gravitational potential energy store when it is placed on a higher shelf.
The faster a runner is moving, the more energy they have in their kinetic energy store. The speed of an object determines the amount of energy in the kinetic energy store.
An object that is stretched or squashed has more energy in its elastic energy store. The amount of energy in the elastic energy store depends on how much the object is stretched or squashed.
Chemical energy stores are found in batteries, foods and fuels. Chemical reactions cause the transfer of energy from the chemical energy store.
Energy cannot be created or destroyed; it can only be stored or transferred. This means that the total energy before and after a change or transfer remains the same. This is known as the Law of Conservation of Energy.
When energy transfers take place in a system:
Total energy before = Total energy after
Energy can be lost from a system by being transferred to the surroundings. This is called energy dissipation (or energy waste). Energy becomes stored in less useful ways.
Energy is usually dissipated to the surroundings by heating. For example, when a bulb is lit, it releases heat energy into the surroundings. This heat energy is not necessary for the bulb’s main function, so it is considered wasted energy.
Summary: