Diffusion
What is diffusion? Diffusion in gases Diffusion is faster in gases than in liquids.
Diffusion is the process by which particles of one substance move through the particles of another substance. Diffusion is how smells travel through the air and how strong liquids become weaker when added to water.
Diffusion happens by itself when the particles move from a place where there are many of them to a place where there are fewer of them. This is called moving from an area of high concentration to an area of low concentration.
The easiest kind of diffusion is when different gases diffuse through each other. As gas particles are very spread out, they can move and vibrate quickly. The gas spreads to areas of the room where its concentration is lower. This continues until the gas is evenly distributed around the entire room.
For example, it happens when a perfume smell spreads throughout the air in an enclosed area.
Diffusion in liquids Brownian Motion What factors affect diffusion?
Diffusion is the movement of particles from where they are more crowded to where they are less crowded until they are evenly spread out. This happens because particles have energy that makes them move and hit each other randomly.
For example, when you mix two liquids, they diffuse into each other. Blackcurrant squash has a lot of particles in a small space. When you add water to the squash, the particles spread out and the squash becomes less strong.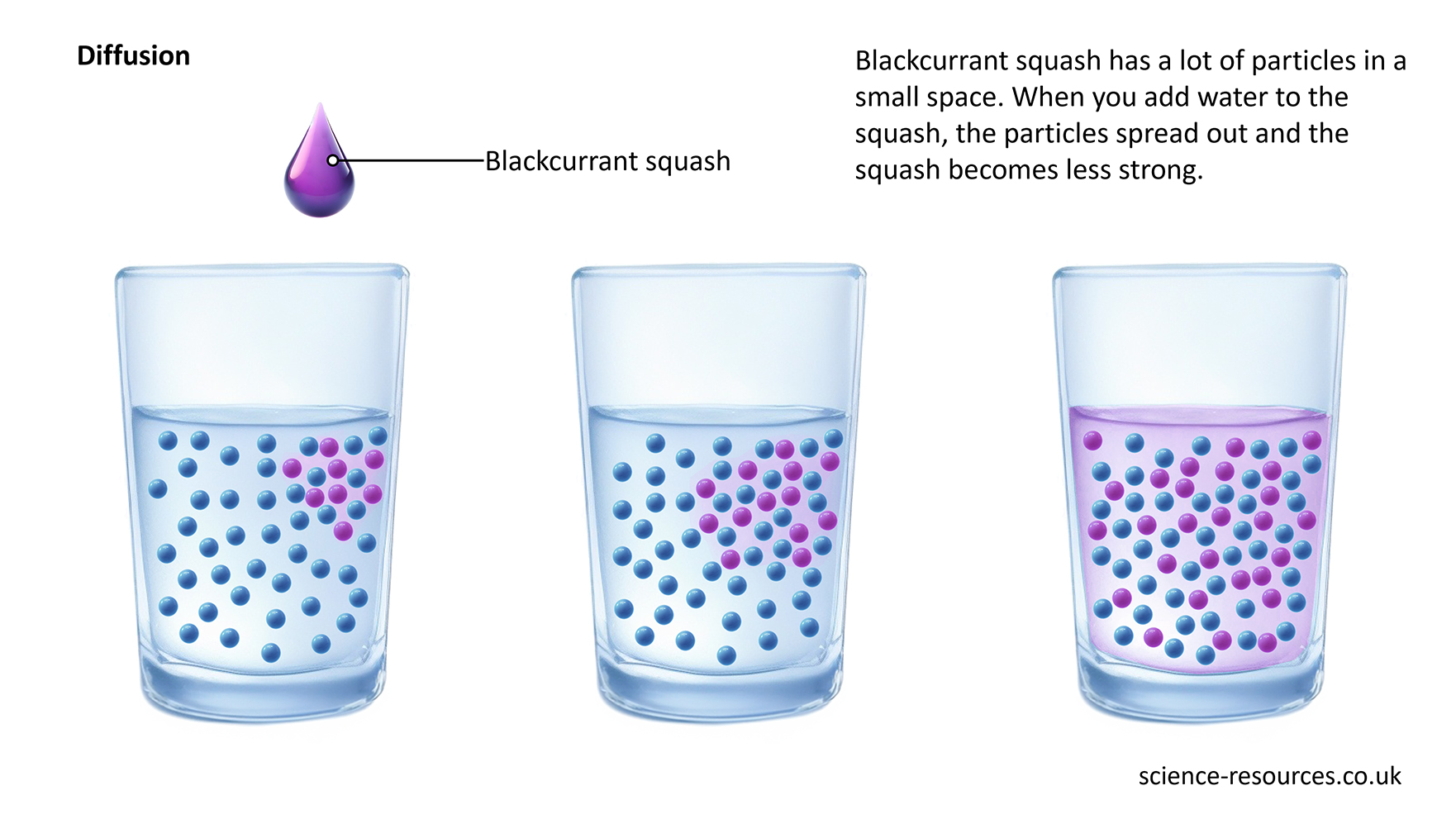
Brownian motion refers to the random movement of particles in gases due to their collisions with other atoms or molecules.
When particles are free to move around, they can collide with other moving particles in the air. These collisions cause the particles to particles change their direction and speed at random.
The rate of diffusion depends on a number of factors:
Summary: