Fractional distillation of crude oil
When crude oil reaches the refinery it is a thick black, smelly liquid.
In this form, it is not much use to anyone. Crude oil contains mixture of hydrocarbons.
At the refinery these are separated
into fractions which are more useful.
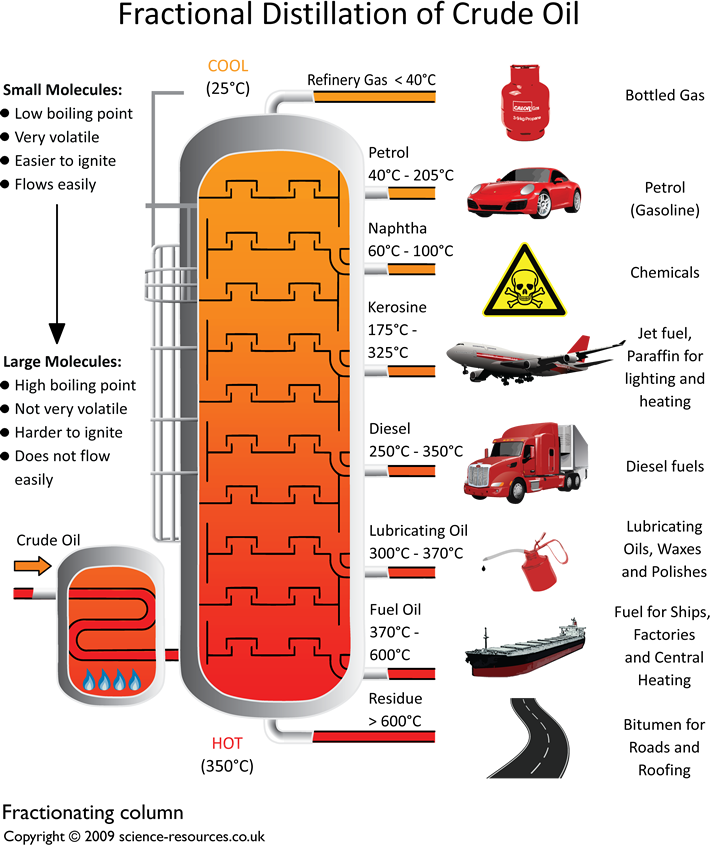
This is done by a process called fractional distillation. This process separates compounds by using the difference
in boiling points. See diagram below. A
Fractionating Column Picture: Yokkaichi oil refinery, Mie Prefecture, Japan Click here to download an A3 printable version of this Crude oil enters the fractionating column as gas.
The column is quite hot at the bottom and cooler at the top.
This difference in the temperature up and down the column sorts the
different fractions from each other. The larger hydrocarbons,
with the high boiling points, turn back into
liquids at the base of the column and the smaller
hydrocarbons stay as gases.
They rise up the column and condense at different levels, as shown in the above diagram. At
the top of the column there are a number of hydrocarbons with low boiling points - between
20ºC and 70ºC. These remain as gases. The discovery of the the crude oil has
played a very big part in the development of modern life. It provides
the fuel for most of today's transport as well as the raw material for making
various chemical like PLASTICS. There are a few things
you must know about hydrocarbons! As the size of the hydrocarbon molecule increases: The boiling
point increases Becomes less flamable Becomes more
viscous Becomes less
volatile Gets less volatile (doesn't evaporate so easily). How does fractional distillation of crude oil work







![]()
Q1: What is crude oil? A1: Crude oil is a thick, black, smelly liquid that contains a mixture of hydrocarbons. Q2: Why is crude oil separated into fractions? A2: Crude oil is separated into fractions because in its raw form, it is not very useful. The fractions are more useful for various applications. Q3: What is the process used to separate crude oil into fractions? A3: The process used to separate crude oil into fractions is called fractional distillation. Q4: How does fractional distillation work? A4: Fractional distillation works by separating compounds based on their boiling points. Crude oil is heated, and the different hydrocarbons are separated as they condense at various levels of the fractionating column. Q5: What happens to larger hydrocarbons during fractional distillation? A5: Larger hydrocarbons, which have higher boiling points, condense back into liquids at the base of the fractionating column. Q6: What happens to smaller hydrocarbons during fractional distillation? A6: Smaller hydrocarbons, which have lower boiling points, rise up the fractionating column and condense at different levels. Q7: Why are hydrocarbons with low boiling points found at the top of the column? A7: Hydrocarbons with low boiling points remain as gases and are found at the top of the fractionating column because the temperature decreases from bottom to top. Q8: What is the significance of crude oil in modern life? A8: Crude oil is significant in modern life as it provides fuel for most of today's transport and serves as the raw material for making various chemicals like plastics. Q9: What happens to large hydrocarbons after distilling crude oil? A9: After distilling crude oil, there is often a surplus of large hydrocarbons called alkanes. Petrochemists use a chemical process called cracking to break these heavy fractions into smaller, more useful ones. Q10: What are some characteristics of larger hydrocarbon molecules? A10: Larger hydrocarbon molecules have higher boiling points, are darker in color, less flammable, less useful, more viscous, less volatile, and do not evaporate easily.Fractional distillation of crude oil FAQ
Tags: Hydrocarbons, Cracking, Petrol, Ethane, Molecules, Diesel, Alkenes, Catalyst, Crude oil, Fractional distillation, Viscosity, how is crude oil separated, crude oil separation process, how to separate crude oil, separation of crude oil, crude oil separation, industrial distillation column, industrial distillation, fractional distillation of crude oil GCSE, how does fractional distillation of crude oil work, what is crude oil a mixture of, tray distillation column, distillation column trays, sieve tray distillation column, industrial distillation column, oil distillation column, how does fractional distillation of crude oil work 24l, distillation column efficiency, distillation column control