Reactivity series
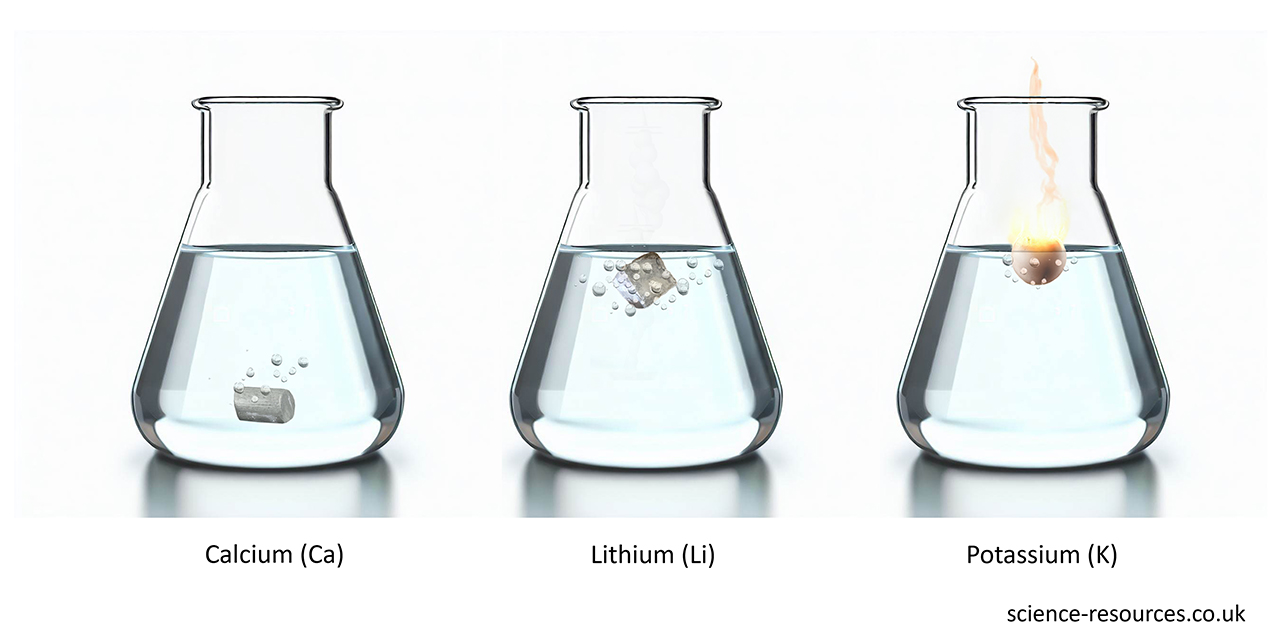
See also: The pH scale The periodic table of elements Reactive metals Observing reactivity 1. Metals and water The table below shows these metals in order of reactivity with water (from most reactive to least reactive): Reactivity Metal Reaction Most reactive Potassium (K) Sparks and a flame produced on contact with cold water ↑ Sodium (Na) Lots of bubbles and movement with cold water Least reactive Calcium (Ca) Tiny bubbles produced slowly with cold water
Reactivity of metals
There are about 95 metals in the periodic table. Metal elements are found on the left and in the middle of the periodic table. Non-metals are found on the right. 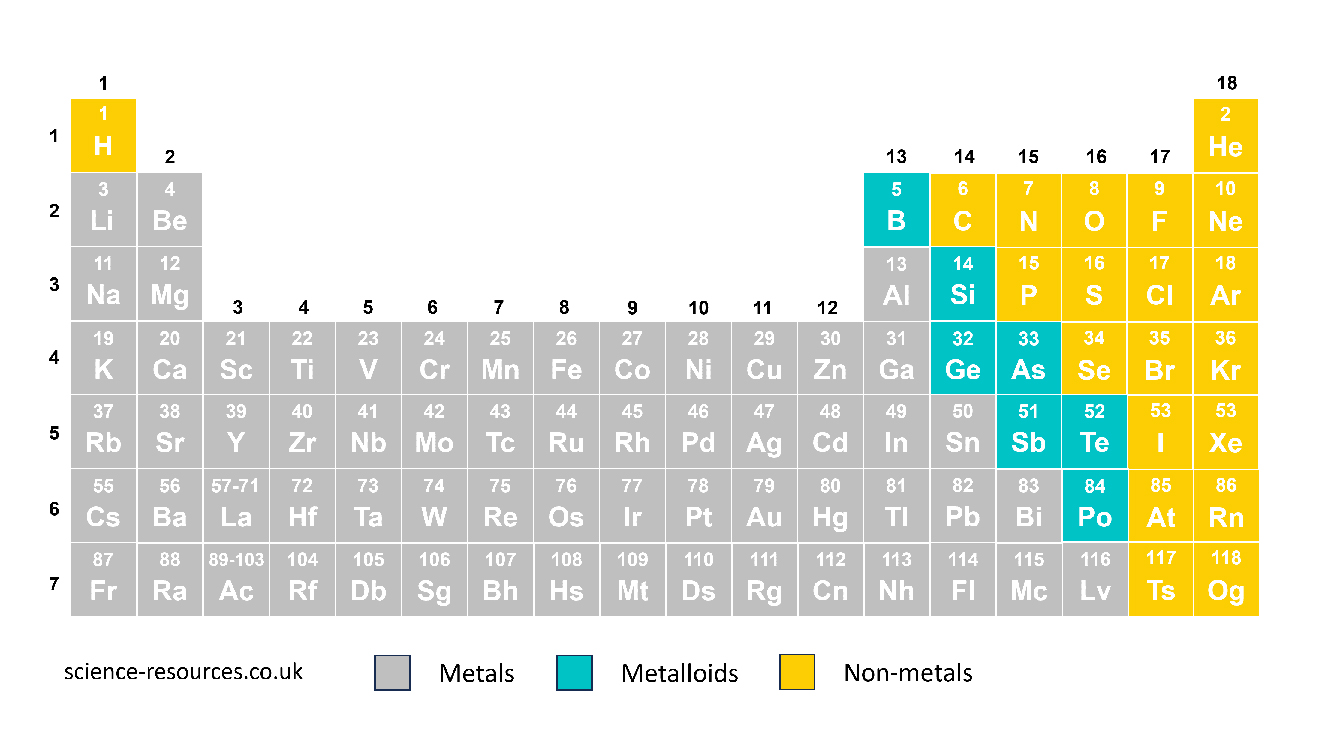
Unreactive metals
Some metals do not react easily. They do not join in chemical reactions very often. For example, copper is not very reactive, so it is good for making water pipes. The water pipes will not react with the water that flows through them.
We can use unreactive metals as they are. For example, platinum is good for jewellery because it does not react with anything, so it always stays shiny.
Some metals are very reactive. They join in chemical reactions very easily.
Lithium, for example, is a very reactive metal. It reacts so quickly that it has to be stored under oil. This stops it from touching the oxygen and moisture in the air. If it did touch the air, it would react very fast.
Lithium makes bubbles of gas when it is put in water. The gas is hydrogen.
Copper is a less reactive metal. It does not react with water at all. It just sinks to the bottom of the beaker.
We mainly use reactive metals in compounds. They have special uses. For example, calcium metal makes hydrogen gas when it reacts with water. But calcium carbonate is in many rocks that we use to build things and make roads.
We can use chemical reactions to see how reactive different metals are. Reactive metals will show changes that we can see, like gas bubbles or flames. Unreactive metals will not show any changes. There are three main types of chemical reactions that we can use to compare metal reactivity. These are reactions of metals with:
Water reacts with some metals, but not with others. Hydrogen gas is made when water and a metal react. The gas can catch fire.
The image below shows three different metals reacting with water:
2. Metals and oxygen When a metal reacts with oxygen, it makes a metal oxide. The table below shows various metals in order of their reactivity with oxygen: Metal Reaction Most reactive Caesium (Cs) Reacts immediately with oxygen in the air. ↑ Magnesium (Mg) Burns in air producing a white flame when heated by a Bunsen burner. ↑ Copper (Cu) Slowly changes colour when heated by a Bunsen burner. Least reactive Gold (Au) Does not react with oxygen. 3. Metals and acid The table below shows various metals in order of their reactivity with acid: Metal Observation Most reactive Potassium (K) Sparks / flame produced when contacts with acid. ↑ Magnesium (Mg) Lots of bubbles and movement on contact with acid. ↑ Zinc (Zn) Tiny bubbles are produced slowly on contact with acid. Least reactive Platinum (Pt) Does not react to acid. Acids do not react with platinum, gold and copper. They are very unreactive metals. They are at the bottom of the reactivity series in the diagram. Lead, tin and zinc are more reactive. They make very small bubbles in a slow reaction with acids. They are above platinum, gold and copper in the diagram. Aluminium, magnesium and calcium react fast with dilute acids. They are above zinc, tin and lead in the diagram. Potassium and sodium react very violently with dilute acids. They make sparks and maybe a small explosion. The reactivity series The reactivity series of different metals and their reactions with oxygen, water and acids.
metal + oxygen → metal oxide
Reactivity
Acids have a pH lower than 7. They make similar observations as water when they react with metals.
Acids do not react with unreactive metals, but very reactive metals like potassium react very quickly and may even make flames.
Reactivity
We can use the reactions of metals with oxygen, water and acid to make a reactivity series of metals.
The metals that react very fast and violently are the most reactive.
The metals that do not show any change are the least reactive.
The reactivity series is a list of the metals in order of reactivity, from the most reactive at the top to the least reactive at the bottom.
See diagram below of the reactivity series of different metals and their reactions: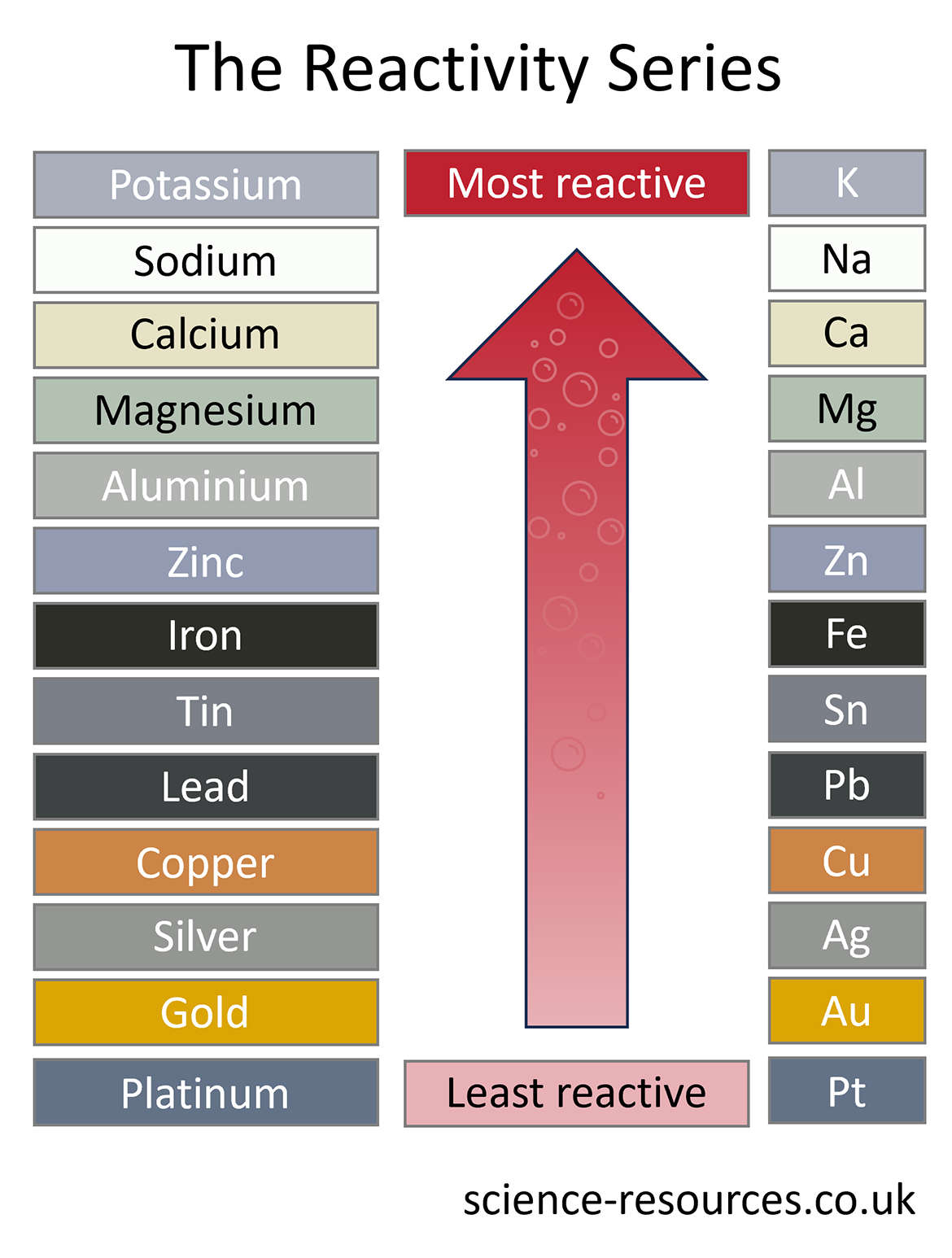
Summary: Q1: What is the reactivity series? Q2: Why is the reactivity series important? Q3: Which metal is the most reactive? Q4: Which metal is the least reactive? Q5: How do metals react with water? Q6: How do metals react with oxygen? Q7: How do metals react with acids? Q8: What happens in a displacement reaction? Q9: Why do some metals need to be stored under oil? Q10: Can the reactivity series change?
Next:
Extracting metals
Displacement reactions
Reactivity Series FAQ
A1: The reactivity series is a list of metals arranged in order of their reactivity, from the most reactive to the least reactive.
A2: It helps predict how metals will react with other substances, such as water, oxygen, and acids. This is useful in understanding chemical reactions and in practical applications like metal extraction.
A3: Potassium is one of the most reactive metals. It reacts very quickly with water and can even produce sparks or flames.
A4: Gold is one of the least reactive metals. It does not react with oxygen or acids, which is why it stays shiny and is used in jewelry.
A5: Metals like potassium and sodium react vigorously with water, producing hydrogen gas and a metal hydroxide. Less reactive metals, like calcium, produce bubbles slowly, while unreactive metals like copper do not react at all.
A6: Reactive metals like magnesium burn brightly when heated in air, forming metal oxides. Less reactive metals, like copper, change color slowly, and unreactive metals like gold do not react with oxygen.
A7: Very reactive metals like potassium can produce sparks or flames when they react with acids. Metals like magnesium produce lots of bubbles, while unreactive metals like platinum do not react with acids.
A8: In a displacement reaction, a more reactive metal can replace a less reactive metal from its compound. For example, zinc can displace copper from copper sulfate solution.
A9: Very reactive metals like lithium and potassium need to be stored under oil to prevent them from reacting with moisture and oxygen in the air.
A10: The reactivity series is based on experimental observations and is generally consistent. However, new discoveries and more precise measurements can refine our understanding of metal reactivity.