Density
Density Arrangement of Particles A particle model of a solid (Note: Particles are all touching) Liquids A particle model of a liquid (Note: The particles in a liquid are closely spaced and randomly arranged.) Gases do not have a fixed shape because the particles can move freely in all directions. They can fill up their container and flow to take its shape. Gases can also be squeezed easily because there is a lot of space between the particles. A particle model of a gas (Note: The particles in a gas are widely spaced and randomly arranged.)
To understand density, it is important to first know the different states of matter: solids, liquids and gases. Let’s look at the arrangement of particles in these three states.
Solids
The particles in a solid are held together by strong forces that keep them in fixed places, in a regular pattern. The particles are very close and only shake around these fixed places. They cannot move around.
Solids have a fixed shape and do not flow like liquids or gases because the particles are in fixed places.
The image below shows the arrangement of particles in a solid.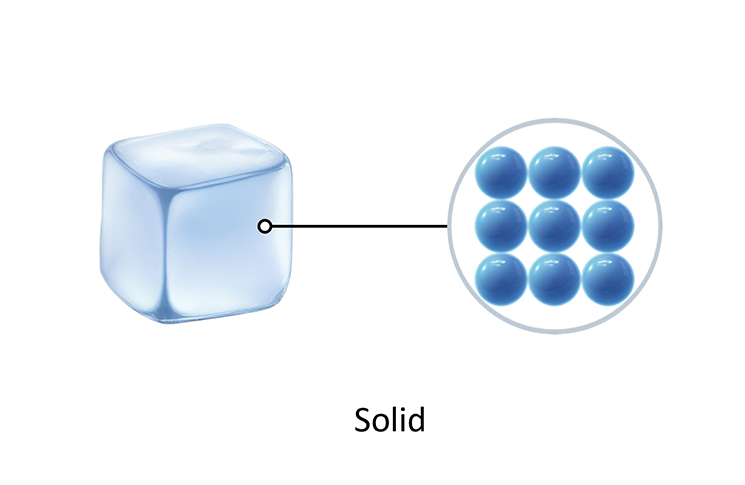
The particles in a liquid are still close together, but they are randomly arranged. They can vibrate and also move around.
Liquids do not have a fixed shape and can flow because the particles can move and vibrate. This means that a liquid will fit the shape of the bottom of its container. Like a solid, liquids cannot usually be squeezed because there is hardly any space between the particles.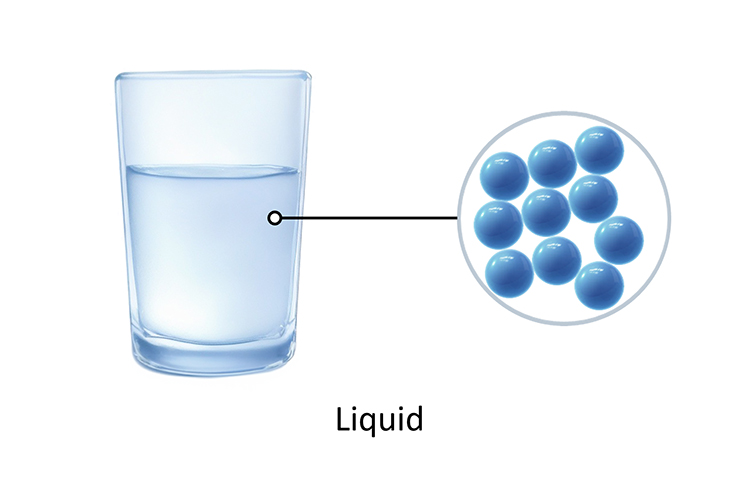
Gases
The particles in a gas are so energetic that they can break free from the forces that try to keep them together. This makes the particles in a gas spread out and move in any direction. The particles in a gas do not shake - they move fast in straight lines, randomly. This means they can hit each other and the walls of any container they are in.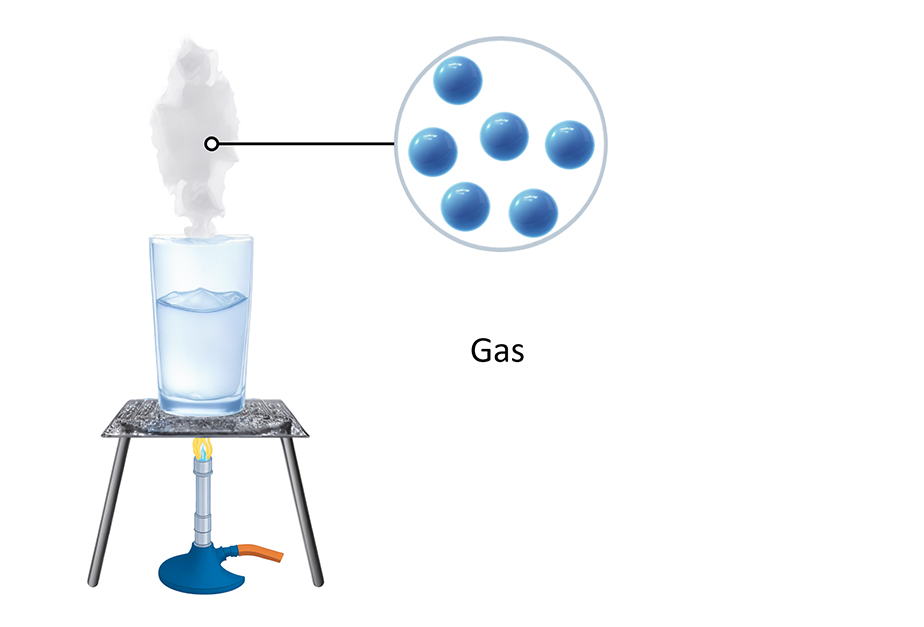
Understanding Density The cube on the right is much heavier than the cube on the left, even though they are both the same size. This is because the cube on the right has a much higher density. Calculating density Density can be measured using a variety of different units, but the most commonly used units are kilograms per cubic metre (kg/m³) and grams per cubic centimetre (g/cm³). Or Where: For example, if the cube on the right has a volume of 10 cm³ and a mass of 50 g, therefore: And the cube on the left has a volume of 10 cm³ and a mass of 5 g, therefore:
Density refers to the mass of an object per unit of volume. For example, let’s compare two blocks with the same volume. 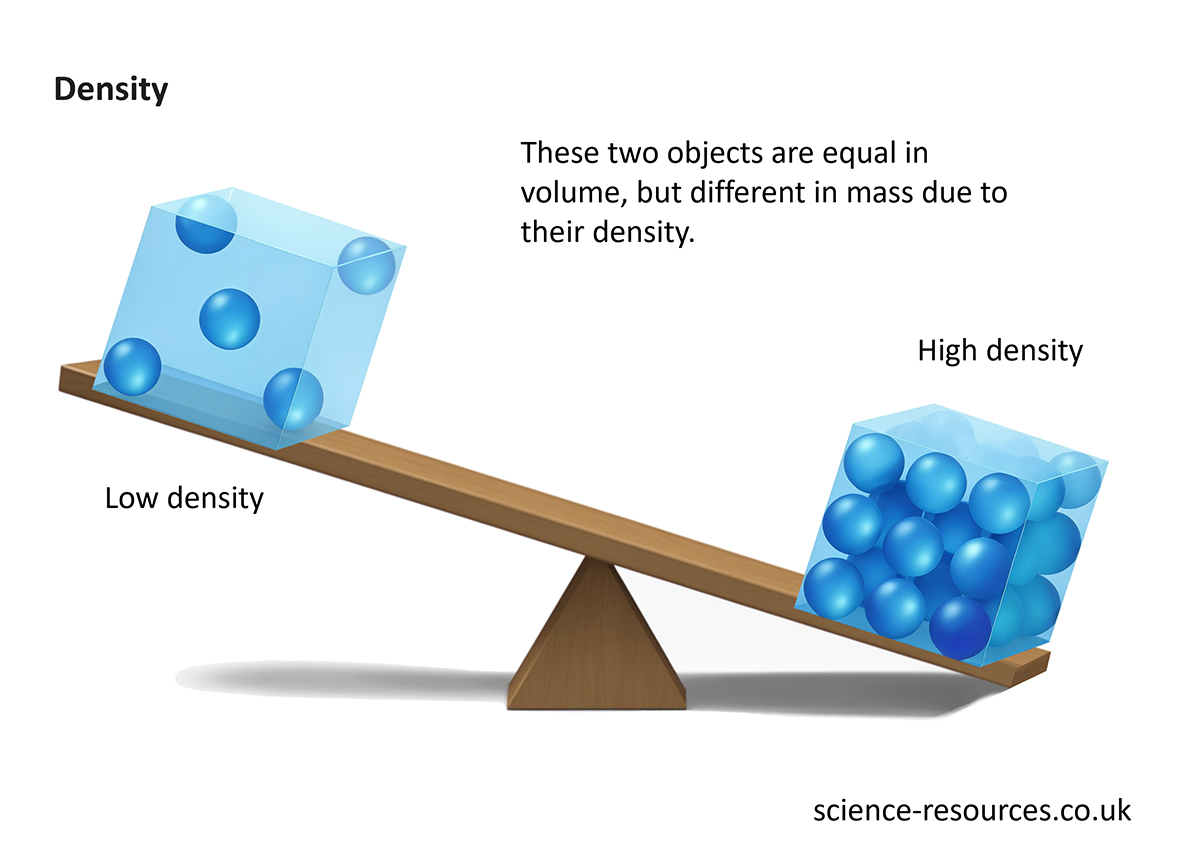
Objects with a higher density feel heavier for their size because more matter is packed into the same space.
To calculate the density of an object, we need:
The equation to calculate density is: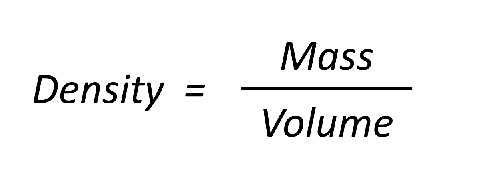
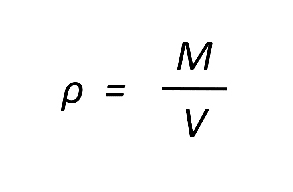
density = mass / volume
density = 50 / 10
density = 5 g/cm³
density = mass / volume
density = 5 / 10
density = 0.5 g/cm³
Summary: