What is water?
See also: What is water made of? Water molecule
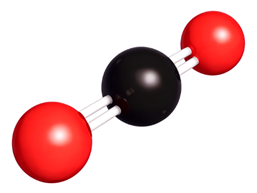
Water is made of molecules. Each molecule has two hydrogen (H) atoms and one oxygen (O) atom.
This means that water’s chemical formula is H2O.
What makes water special? Why is water important? Humans need to drink enough water to replace what we lose though sweat, urine, and faeces.
Water is the most abundant substance on Earth’s surface. It covers more than 70% of the planet in oceans, rivers, lakes and glaciers.
Water has some unique properties:
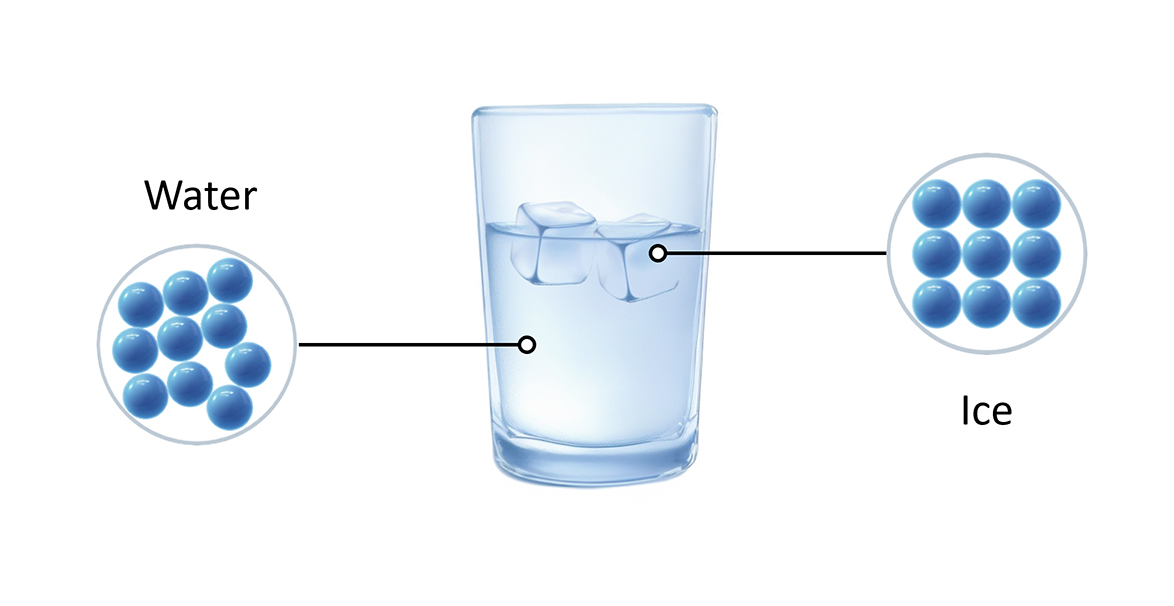
Water is vital to all living things on Earth. Plants use water in photosynthesis to make their food.
About 60% of the human body is water and it does many things, such as:
Summary: Q1: What is water made of? A1: Water is made of molecules, each consisting of two hydrogen (H) atoms and one oxygen (O) atom, giving it the chemical formula H2O. Q2: What makes water special? A2: Water has unique properties: it can be solid, liquid, or gas at normal Earth temperatures, is most dense at 4°C, dissolves many substances, and has high surface tension. Q3: Why is water important? A3: Water is vital for all living things. It is used in photosynthesis, makes up about 60% of the human body, and helps dissolve nutrients, regulate temperature, remove waste, and protect tissues. Q4: How much of Earth’s surface is covered by water? A4: Water covers more than 70% of Earth’s surface in oceans, rivers, lakes, and glaciers. Q5: What are the three states of water? A5: Water can exist as a solid (ice), liquid (water), or gas (vapor). Q6: At what temperature is water most dense? A6: Water is most dense at 4°C. Q7: How does water benefit the human body? A7: Water dissolves nutrients, regulates body temperature, removes waste, and protects tissues, joints, and the spinal cord. Q8: Why do humans need to drink water? A8: Humans need to drink water to replace what is lost through sweat, urine, and feces. Q9: What role does water play in photosynthesis? A9: Water is used by plants in photosynthesis to make their food. Q10: What substances can water dissolve? A10: Water can dissolve many substances, including salts and proteins.
Next:
Changes of state
Solids, liquids, gases
What is water FAQ